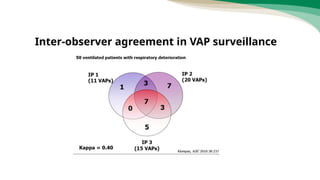
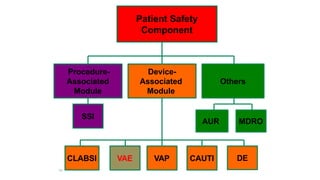
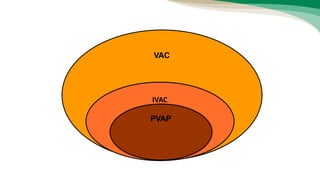
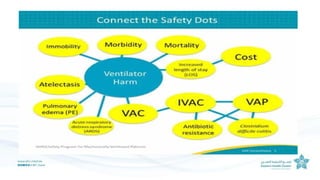
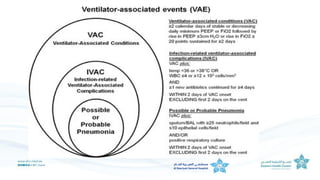
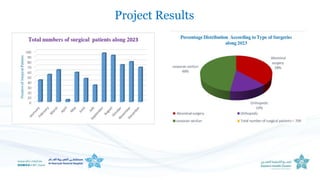
The document outlines the transition from ventilator-associated pneumonia (VAP) surveillance to ventilator-associated event (VAE) surveillance by the CDC, emphasizing a structured algorithm with three tiers: ventilator-associated conditions, infection-related complications, and possible VAP. It discusses methods to lower VAP rates through strict clinical interpretations and introduces the 'iCough' bundle aimed at reducing respiratory infections in postoperative patients. The study concludes that implementing the iCough bundle significantly improves patient outcomes, reduces infection rates, and enhances patient satisfaction.