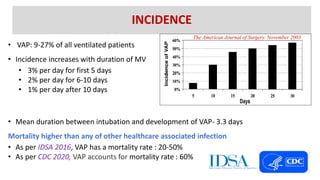
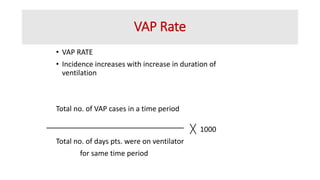
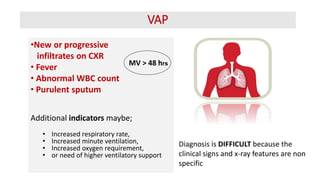
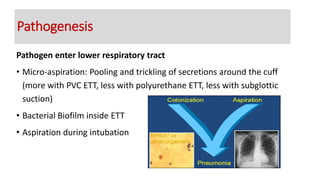
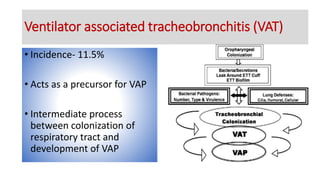
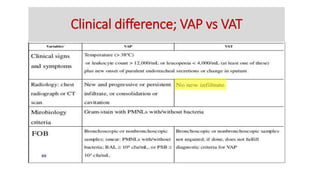
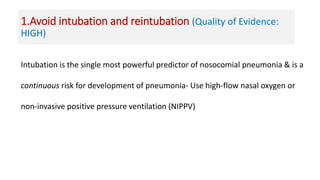
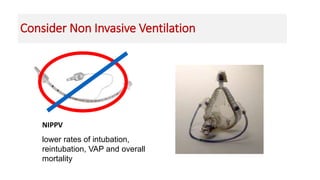
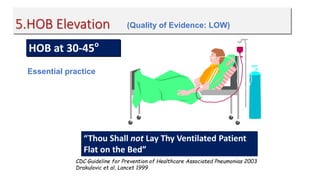
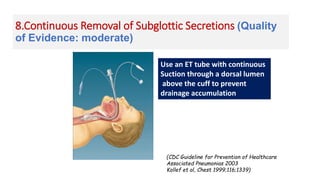
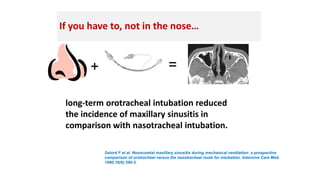
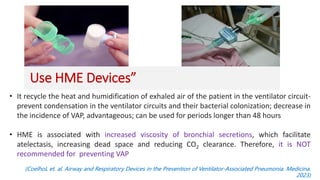
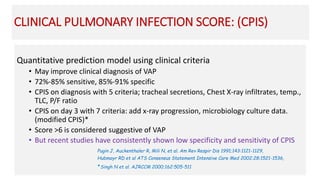
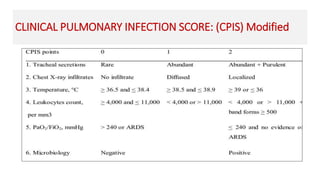
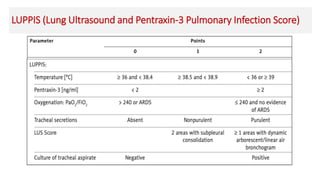
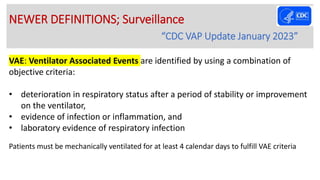
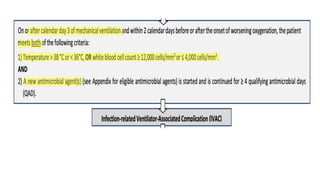
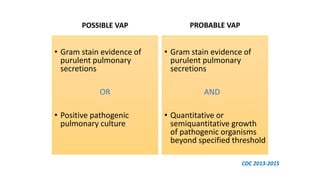
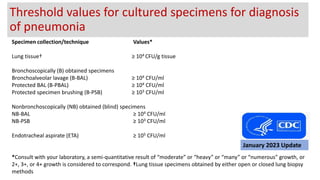
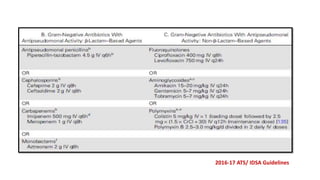
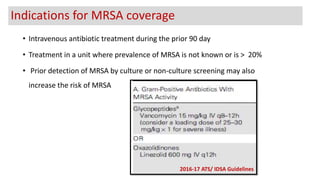
Ventilator-associated pneumonia (VAP) is a common hospital-acquired infection that prolongs mechanical ventilation and ICU stays. It has a high mortality rate of 20-50%. Risk factors include prolonged mechanical ventilation, supine positioning, and use of sedatives. Diagnosis is difficult due to non-specific signs. New tools like LUPPIS aim to aid early diagnosis. Prevention strategies recommended by guidelines include early mobility, oral care, subglottic secretion drainage, and selective decontamination in some settings.