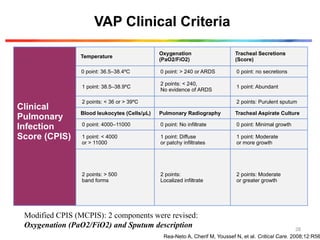
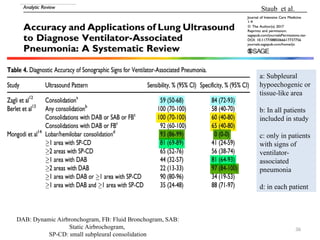
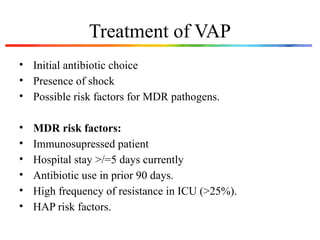
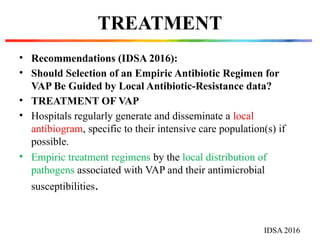
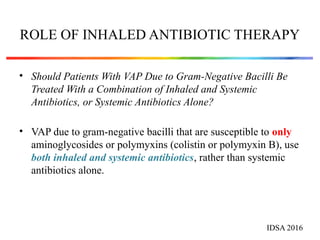
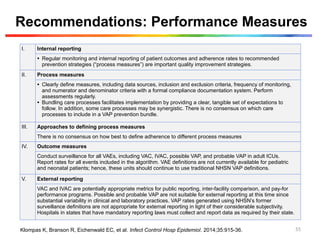
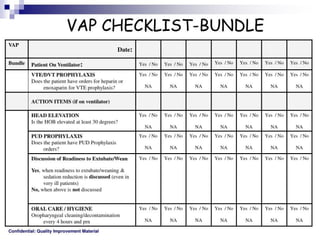
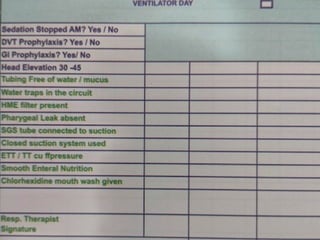
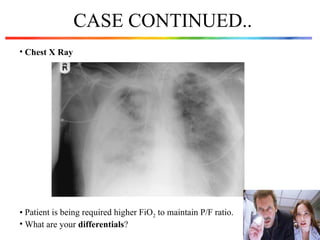
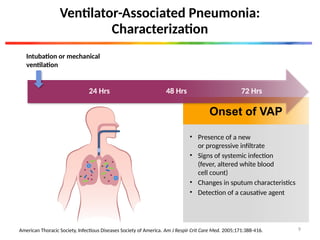
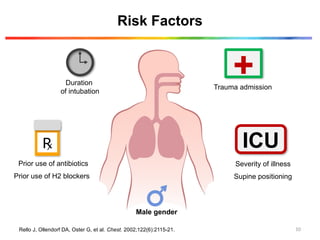
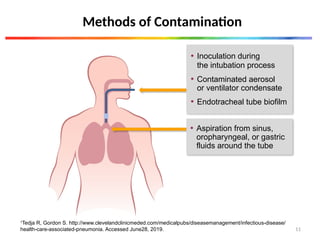
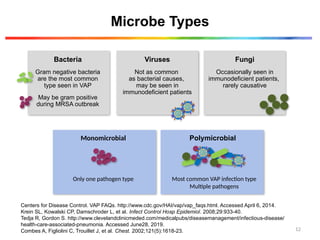
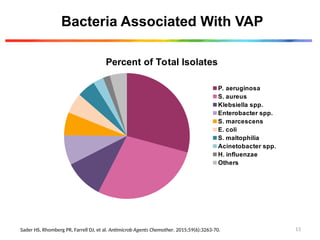
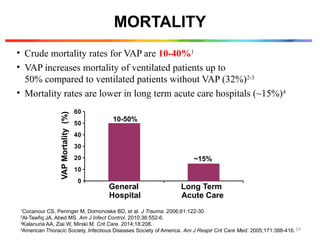
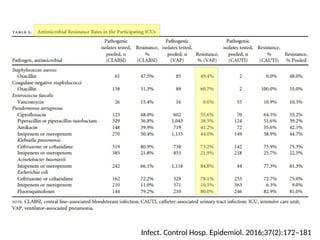
The document discusses a case of a 52-year-old female with ventilator-associated pneumonia (VAP) following a pontine infarct, emphasizing the definition, risk factors, and diagnostic criteria of VAP, which develops in patients on mechanical ventilation for over 48 hours. It highlights the epidemiology, treatment protocols, and costs associated with VAP, noting the necessity for tailored antibiotic therapy based on local resistance patterns. The guidelines established by the CDC and IDSA are recommended for managing VAP to improve patient outcomes.





![Possible Ventilator-Associated Pneumonia
On or after calendar day 3 of mechanical ventilation
and within 2 calendar days before or after the onset
of worsening oxygenation, ONE of the following
criteria is met:
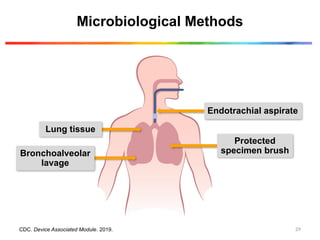
1) Criterion 1: Positive culture of one of the following specimens, meeting quantitative or semi-quantitative thresholds as outlined in pr
requirement for purulent respiratory secretions:
Endotracheal aspirate- ≥ 105
CFU/mL or corresponding semi-quantitative result;
bronchoalveolar lavage- ≥ 104
CFU/mL or corresponding semi-quantitative result;
lung tissue- ≥ 104
CFU/g or corresponding
semi-quantitative result;
protected specimen brush -≥ 103
CFU/mL or corresponding semi-quantitative
result
2) Criterion 2: Purulent respiratory secretions
(defined as secretions from the lungs, bronchi,
or trachea that contain > 25 neutrophils and
< 10 squamous epithelial cells per low power field
[lpf, x100]) plus organism identified from one of the following specimens (to include qualitative culture,
or quantitative/semi-quantitative culture without
sufficient growth to meet criterion #1):
Sputum, endotracheal aspirate, bronchoalveolar
lavage, lung tissue, protected specimen brush
3) Criterion 3: One of the fo
Organism identified from p
of chest tube and NOT from
1) abscess formation or foc
intense neutrophil accumu
and alveoli; 2) evidence of
by fungi (hyphae, pseudohy
3) evidence of infection wit
on respiratory secretions fo
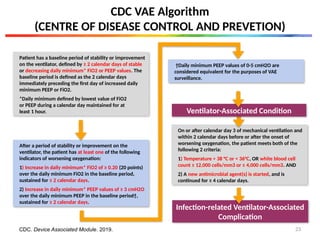
CDC VAE Algorithm
CDC. Device Associated Module. 2019.
Infection-related Ventilator-Associated Complication
24](https://image.slidesharecdn.com/vap-241016064522-3a659558/85/VAP-Ventilator-associated-pnemonia-pptx-22-320.jpg)