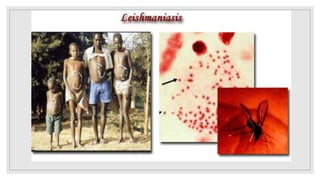
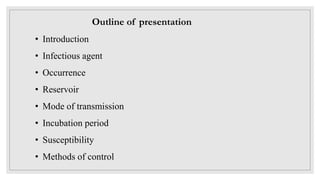
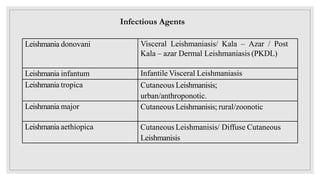
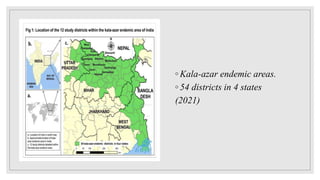
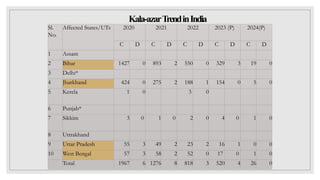
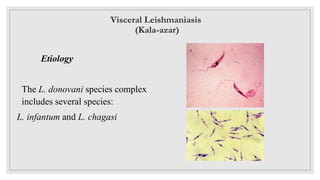
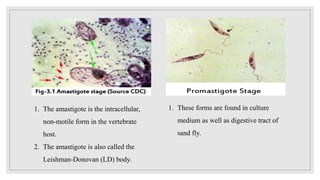
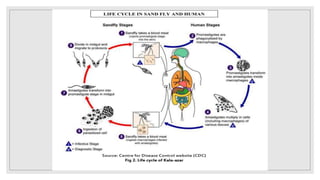
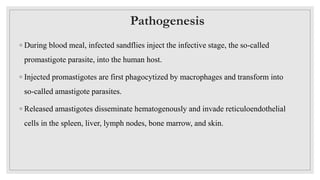
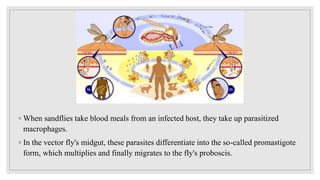
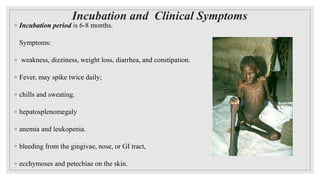
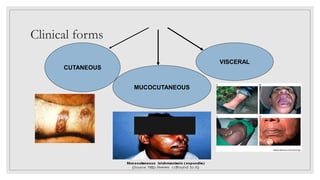
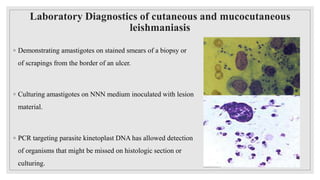
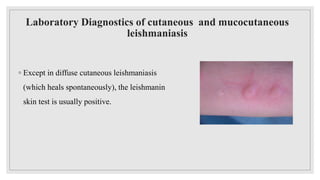
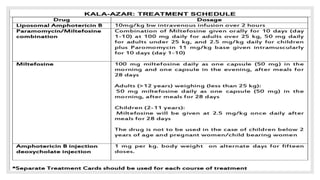
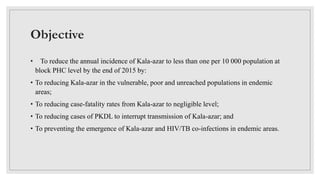
Leishmaniasis is a parasitic disease caused by the Leishmania protozoa, presenting mainly as cutaneous, mucocutaneous, and visceral forms, with kala-azar being the most severe. The disease is primarily transmitted by the sandfly and is endemic in various regions including India, where significant control measures have been implemented since the 1990s, resulting in a notable decline in cases. The Indian government aims to eliminate kala-azar by reducing its incidence to less than one case per 10,000 population through various public health strategies and collaborations with neighboring countries.