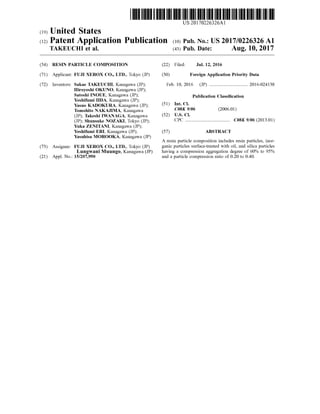
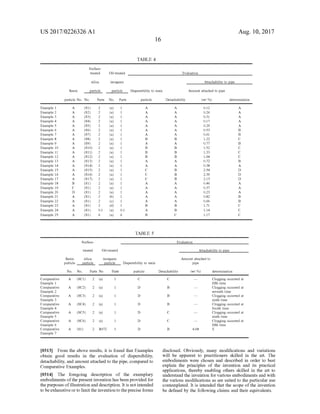
This document describes a resin particle composition containing resin particles, inorganic particles surface-treated with oil, and silica particles. The silica particles have specific properties including a compression aggregation degree of 60-95% and a particle compression ratio of 0.20-0.40. These specific silica particles improve the dispersibility of the oil-treated inorganic particles to the resin particles and increase the lubrication function, preventing fixation of the resin particle composition in pipes during air transportation.