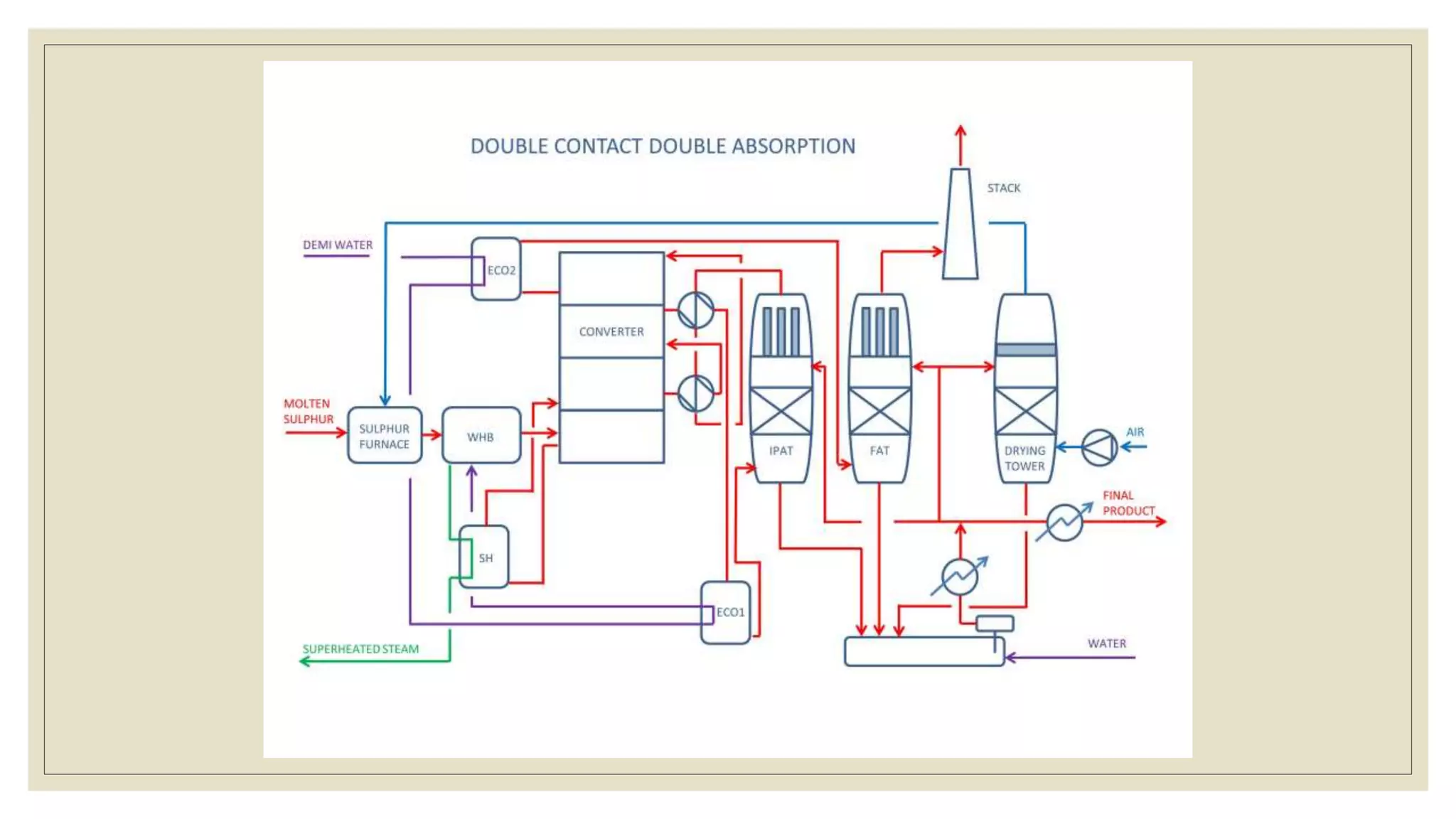
Sulfuric acid is a colorless, oily liquid with the molecular formula H2SO4. It is produced via the contact process, which involves three steps: burning sulfur to produce sulfur dioxide, oxidizing the sulfur dioxide to form sulfur trioxide, and hydrating the sulfur trioxide to produce sulfuric acid. Vanadium pentoxide is used as the catalyst for the oxidation reaction. Major engineering challenges in the process include designing multistage converters for the exothermic oxidation reaction and improving heat transfer for the hydration reaction. Sulfuric acid has many industrial uses including fertilizer production, explosives manufacturing, and making detergents and plastics.