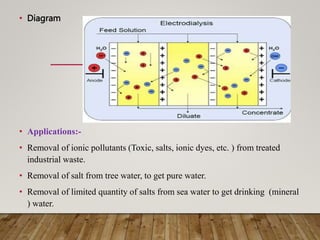
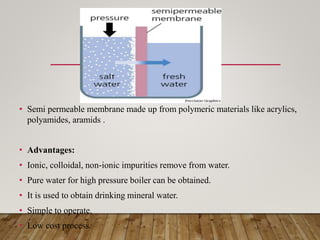
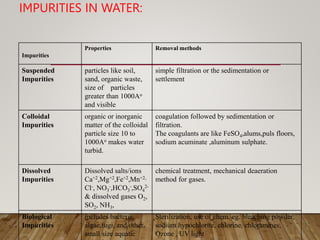
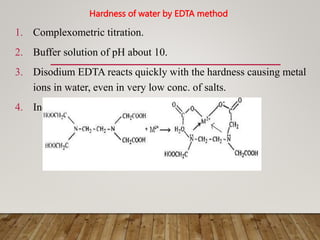
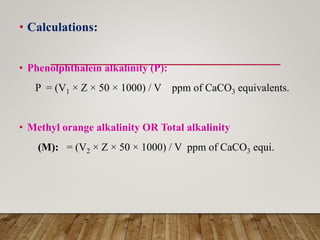
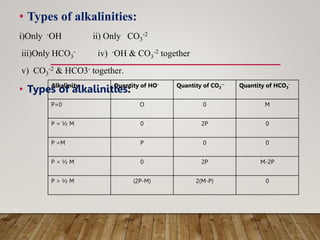
This document discusses various impurities found in water and their removal methods. It describes suspended, colloidal, dissolved, and biological impurities and filtration, coagulation, chemical treatment, and sterilization methods. It also discusses hardness in water, including temporary and permanent hardness and their removal by boiling or other chemical methods. EDTA titration and alkalinity determination methods are outlined. Issues caused by hard water in boilers like corrosion, priming, foaming, sludge, scale, and caustic embrittlement are summarized along with their prevention.



















![Internal treatment of boiler scale.
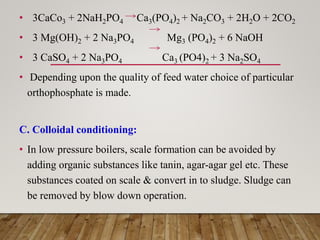
• Principle: - “Chemicals added for internal treatment either does
not allow the scale formation to take place or they attack on the
previously formed scales to convert them into sludge. Or
dissolve the scales.”
A. Calgon conditioning:
• Calgon is sodium hexametaphosphate (NaPO3)6 or
Na2[Na4P6O18). This substance forms highly soluble
coordination complexes with Ca++ Mg++, Fe++ and thus scale
formation is prevented. The optimum pH required for complex
formation is 9.0 to 10.5
• Na2[Na4P6O18) 2Na+ + [Na4P6O18)
• Ca++ + [Na4P6O18]-2 2Na+ + [CaNa2P6O18]-2
(Soluble complex)](https://image.slidesharecdn.com/unit1ppt-231004152441-bdd384c8/85/unit-1-ppt-pptx-24-320.jpg)