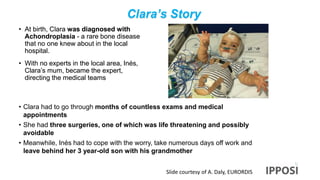
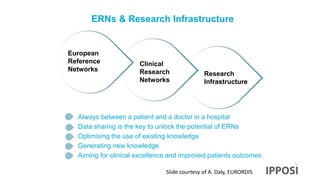
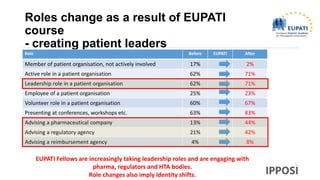
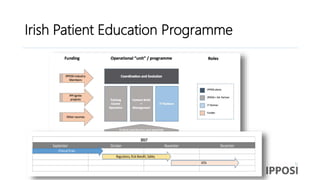
The document discusses the activities and goals of IPPOSI, a patient-led organization in Ireland focusing on rare diseases and health innovation. It highlights Clara's story as a case study of the challenges faced by patients with rare conditions and the need for greater patient involvement in healthcare decisions. The document emphasizes the importance of coordinated research efforts, patient empowerment, and policy development to address the needs of individuals affected by rare diseases.