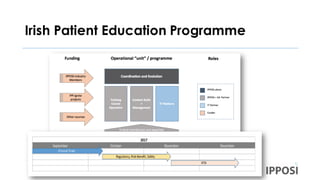
The document discusses the importance of patient involvement in health research and innovation, highlighting the role of organizations like IPPOSI in advocating for patient access and meaningful participation. It outlines key actions to enhance patient engagement in research design, regulatory affairs, and health technology assessments, while also emphasizing the shift in leadership roles among patient advocates. Additionally, it addresses the need for education and support for both patients and researchers to improve research quality and foster collaboration.