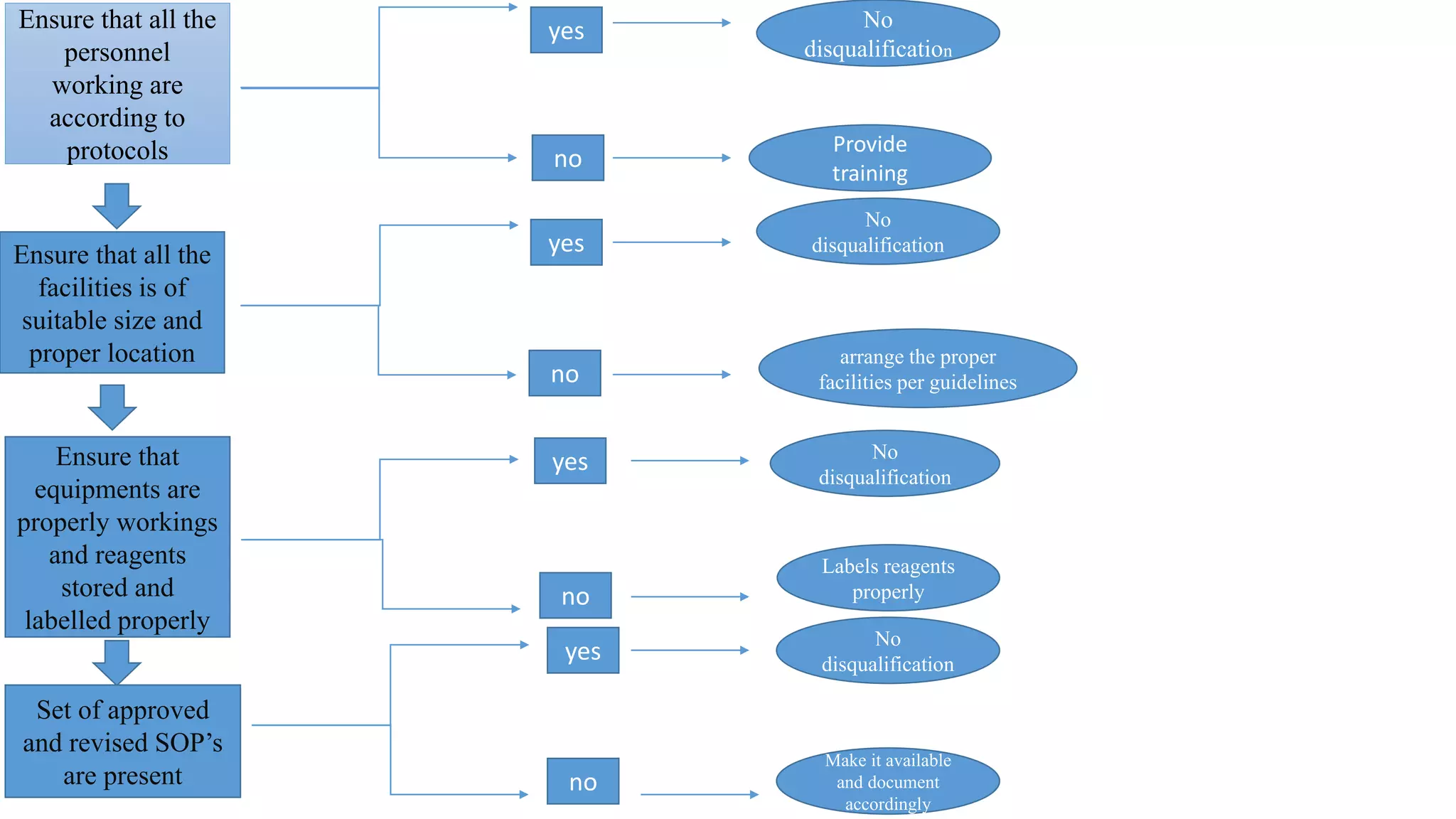
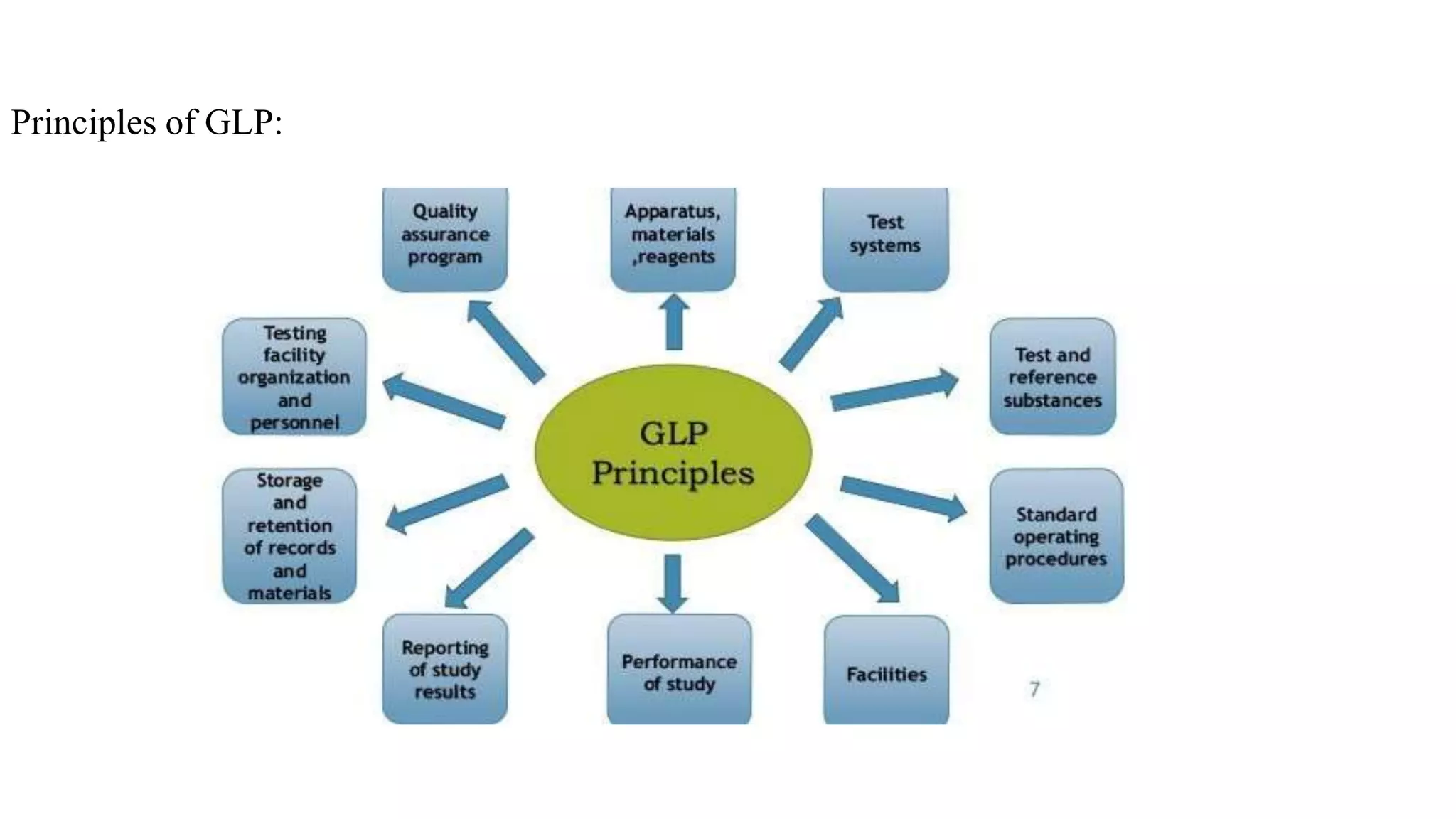
Good Laboratory Practices (GLP) were established by the FDA in 1978 in response to poor laboratory practices that compromised research integrity. GLP aims to ensure the quality and validity of test data for non-clinical studies by providing guidelines for personnel, facilities, equipment, and testing procedures. Compliance with GLP is essential for supporting applications for FDA-regulated products, promoting public health safety, and facilitating international acceptance of test results.










![SUBPART G- PROTOCOL FOR CONDUCT OF A NON CLINICAL LABORATORY STUDY
a. Protocol-each study shall have an approved written protocol that clearly indicates the objectives and all
methods foe the conduct of the study. A descriptive title and statement of the purpose of the study.
Identification of the test and control articles by name, chemical abstract number, or code number The name
of the sponsor and the name and address of the testing facility at which the study is being conducted.The
number, body weight range, sex, source of supply, species, strain, sub strain, and age of the test system The
procedure for identification of the test system. A description of the experimental design, including the
methods for the control of bias.
b. Conduct of a non clinical laboratory study-in accordance with the protocol .
SUBPART H-I [RESERVED]
SUBPART J-RECORDS AND REPORTS
a. Reporting of non clinical laboratory study results
b. Storage and retrieval of records and data
c. Retention of records](https://image.slidesharecdn.com/goodlaboratorypracticesglp-230126034407-756b0feb/75/GOOD-LABORATORY-PRACTICES-GLP-pptx-12-2048.jpg)