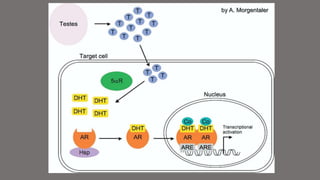
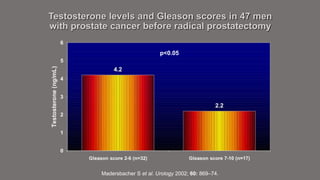
The document discusses testosterone replacement therapy (TRT) and prostate cancer. It notes that while traditional views held that high testosterone led to rapid cancer growth, recent evidence challenges this. Studies found no association between endogenous hormone levels and cancer risk. The saturation model suggests maximal cancer growth occurs at relatively low testosterone levels. Accumulating evidence also links low testosterone with higher-risk cancer features. TRT studies up to 3 years found similar cancer detection rates to screening. While long-term safety data is still needed, available evidence suggests TRT may be considered for selected prostate cancer patients with symptoms of hypogonadism after obtaining informed consent.