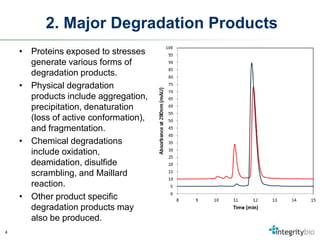
The document outlines ten major factors critical for successful protein formulation development, emphasizing the importance of understanding each protein's unique characteristics and the relevant stresses they experience. It highlights how proteins can degrade under various physical and chemical stresses, generating different degradation products that must be addressed in formulation studies. Integrity Bio, a contract development and manufacturing organization, specializes in formulating biologics and has extensive experience with over 150 products including vaccines and proteins.