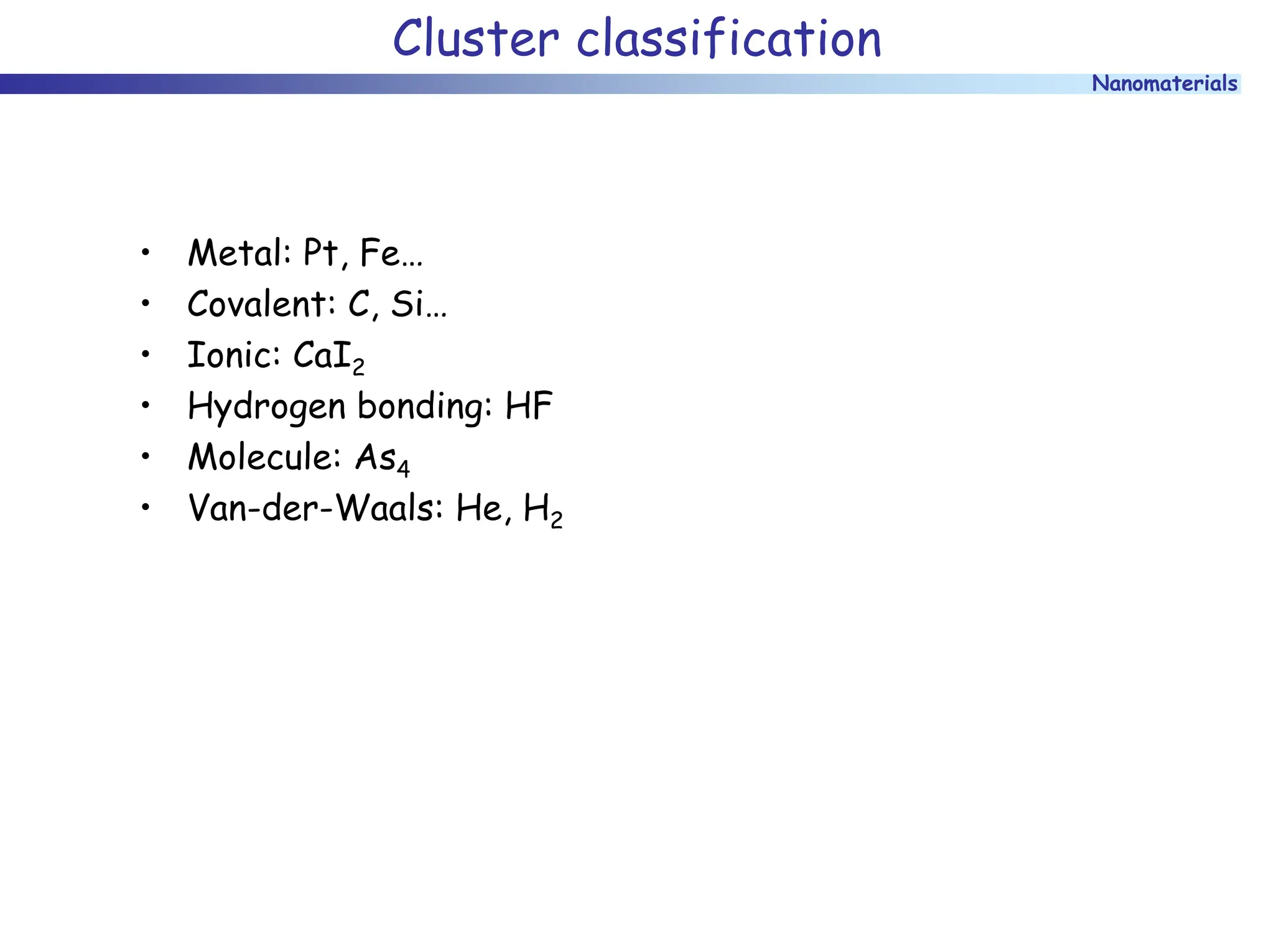
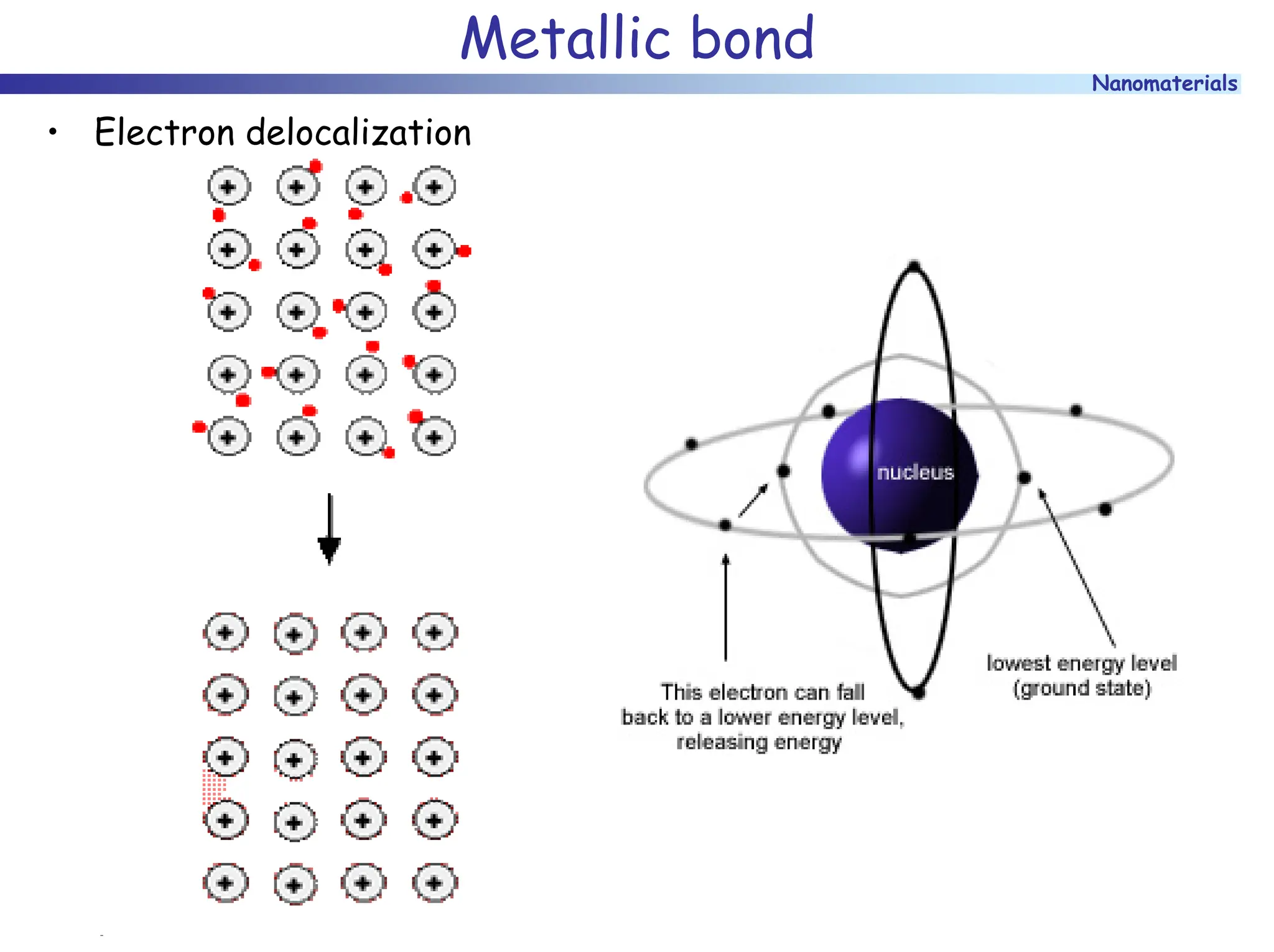
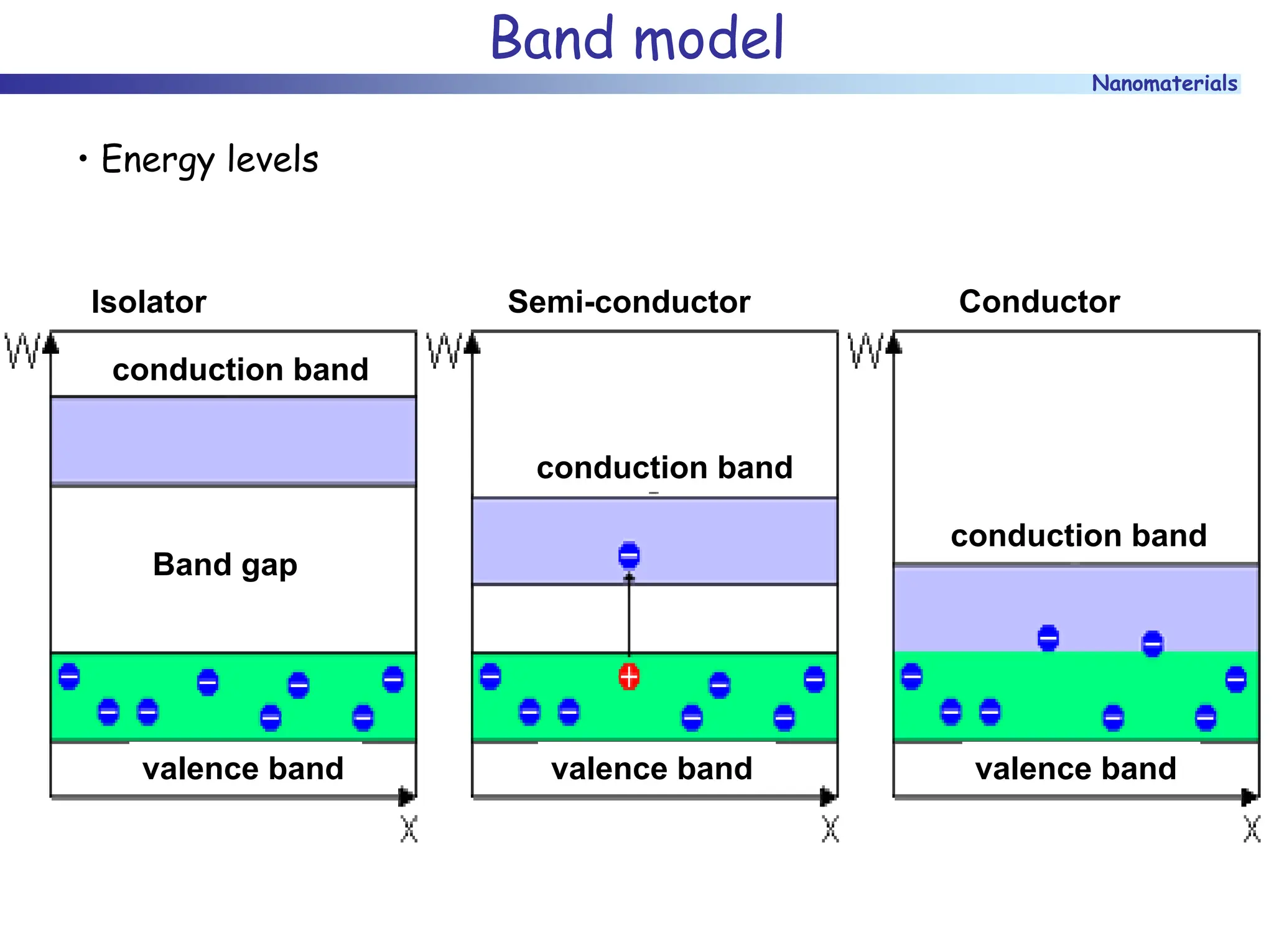
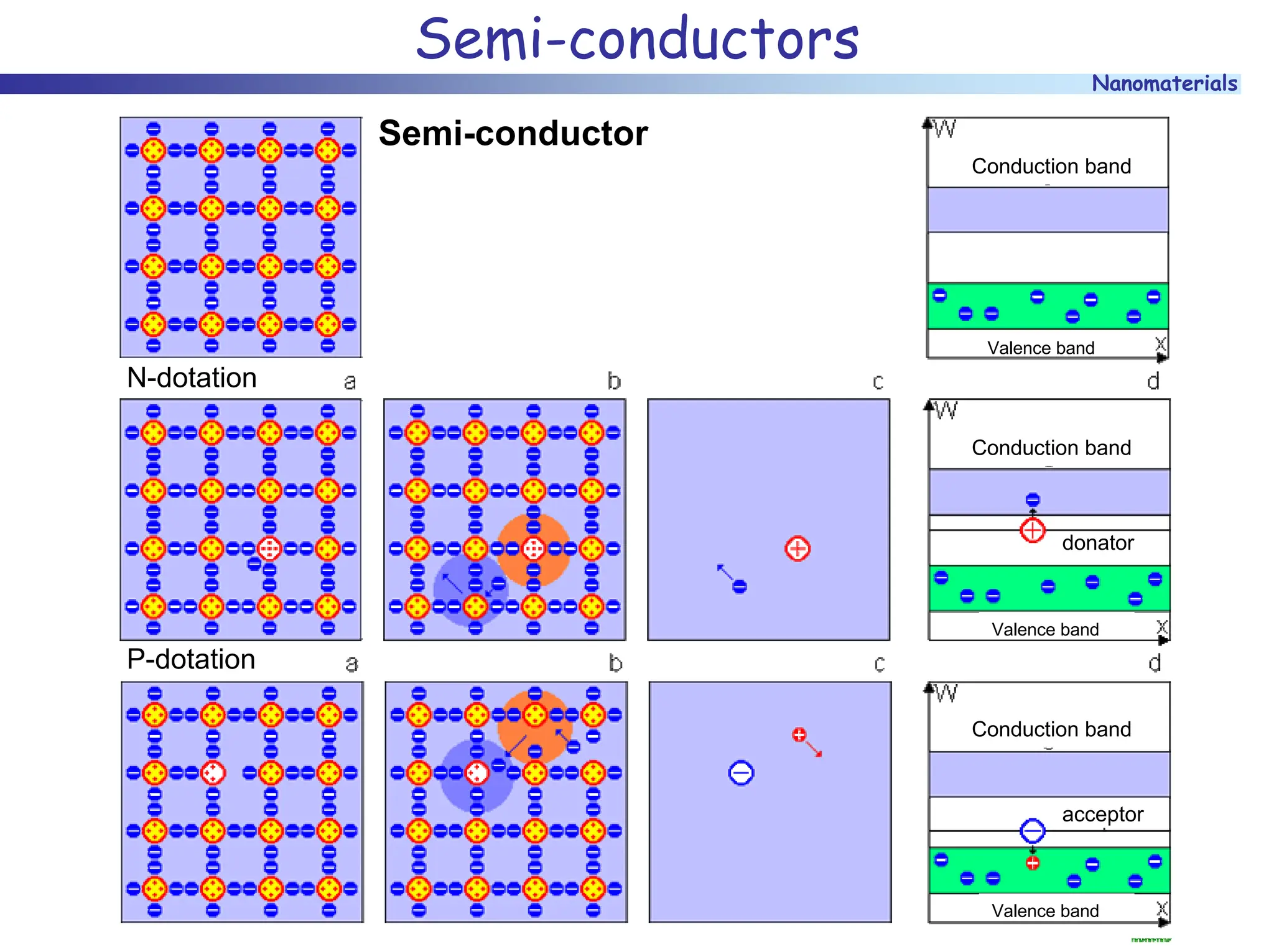
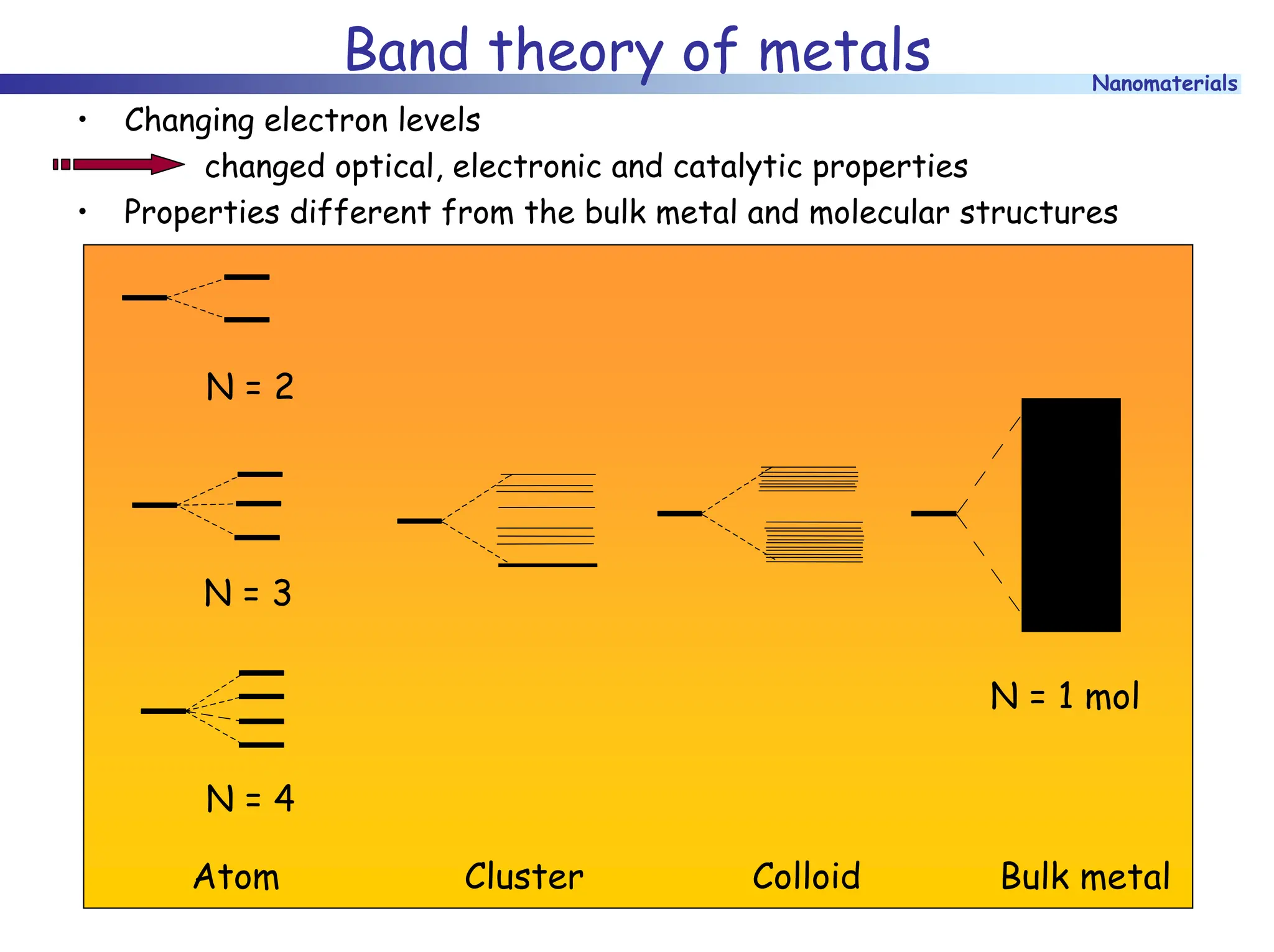
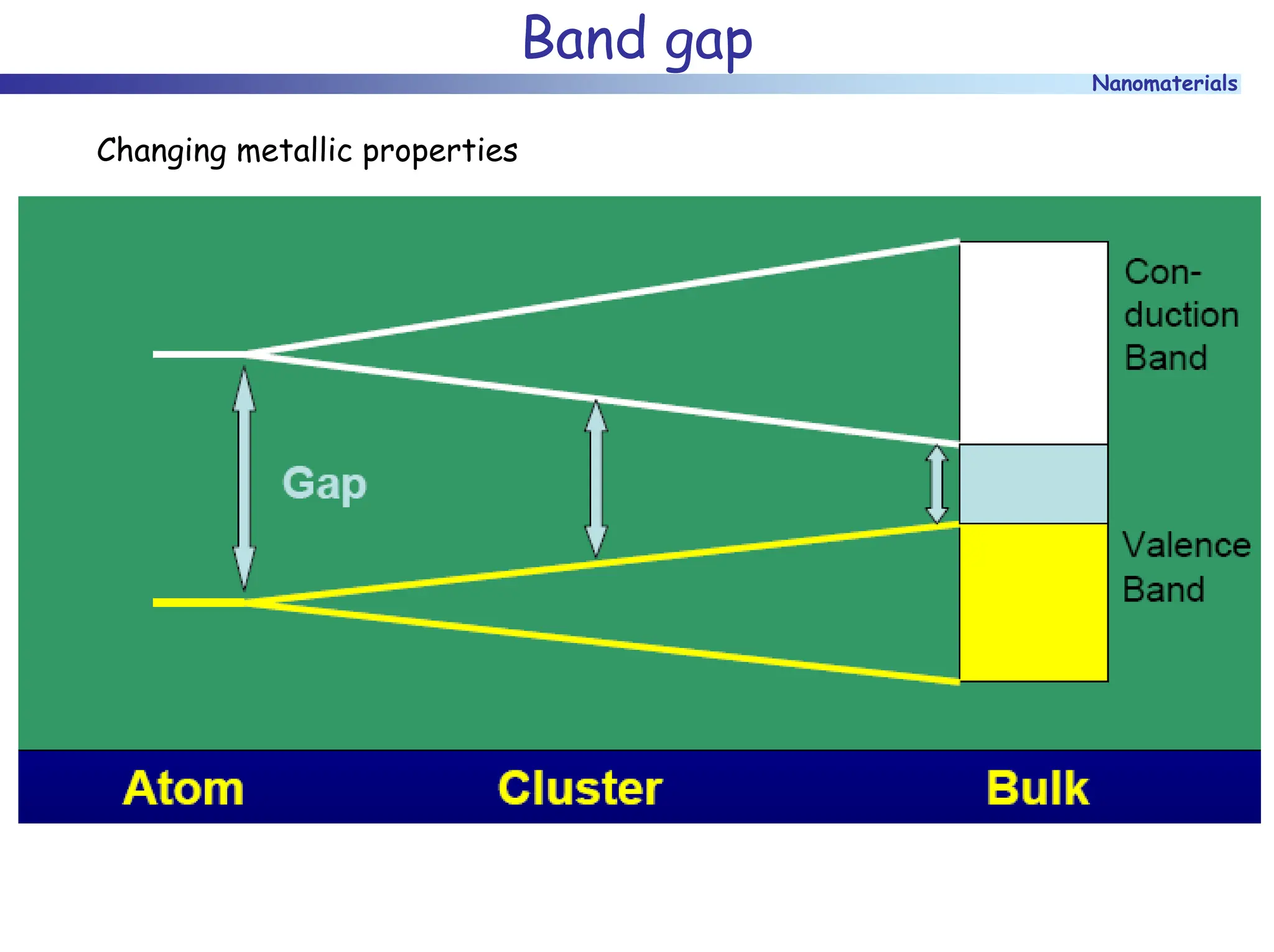
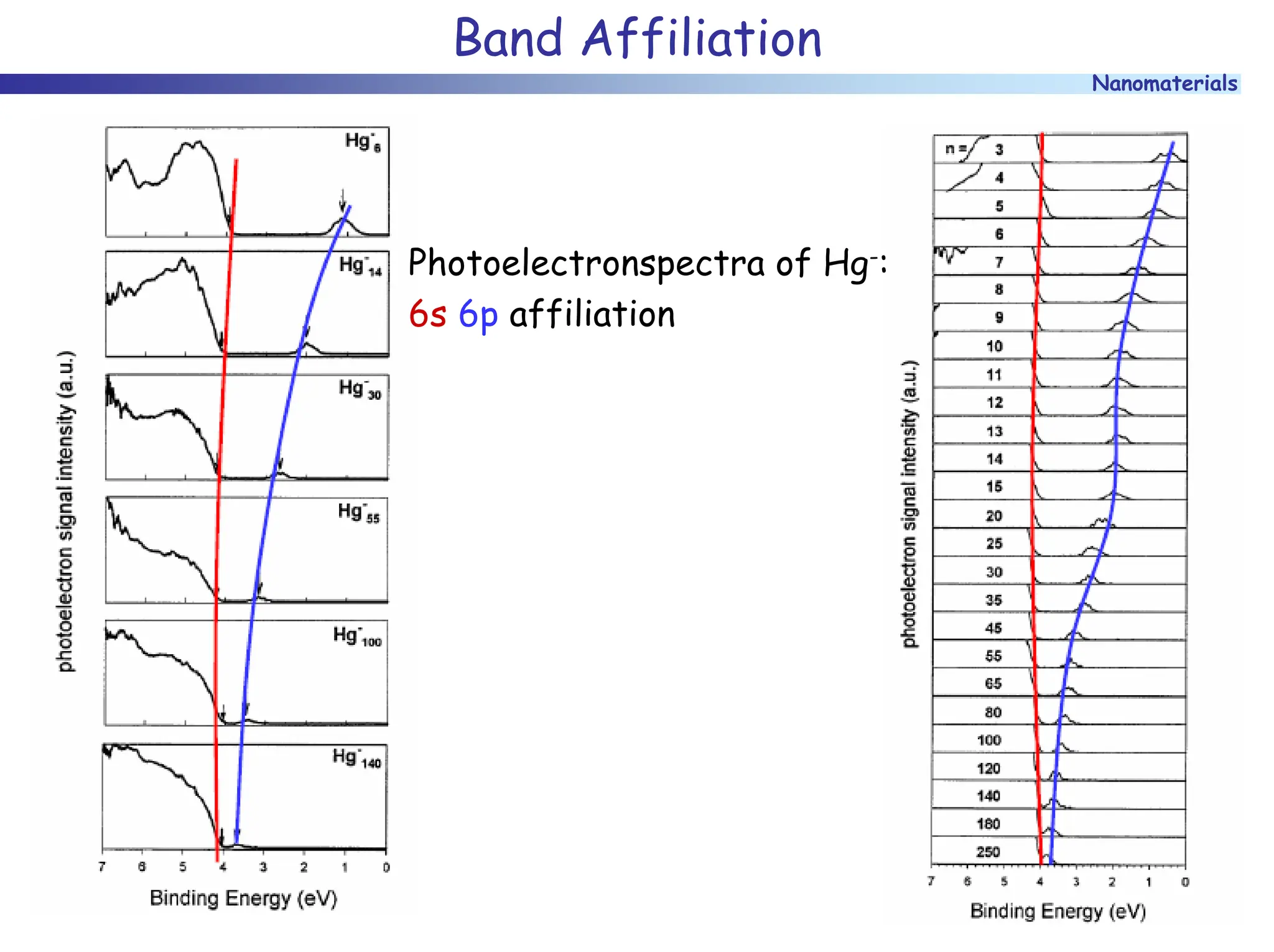
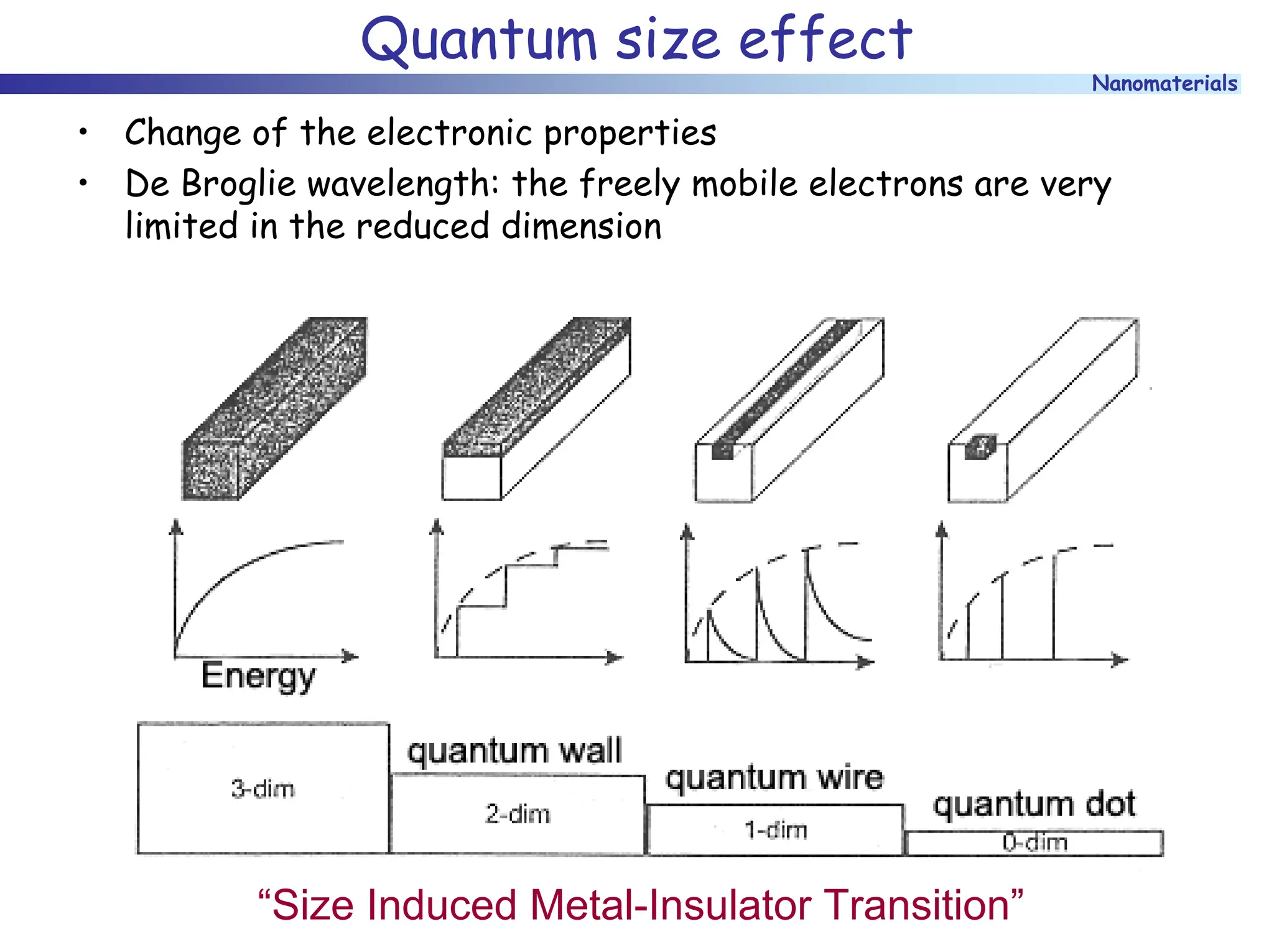
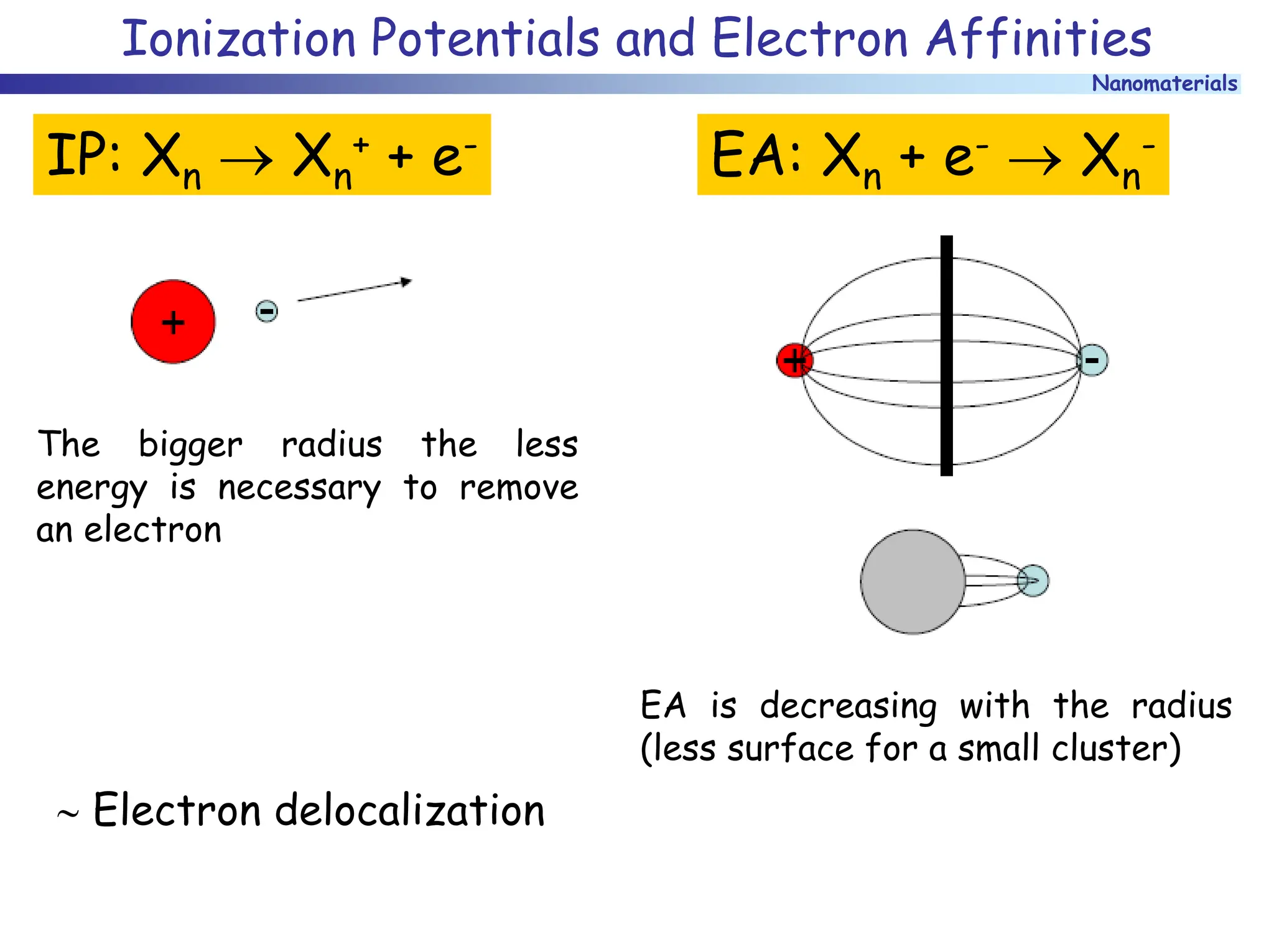
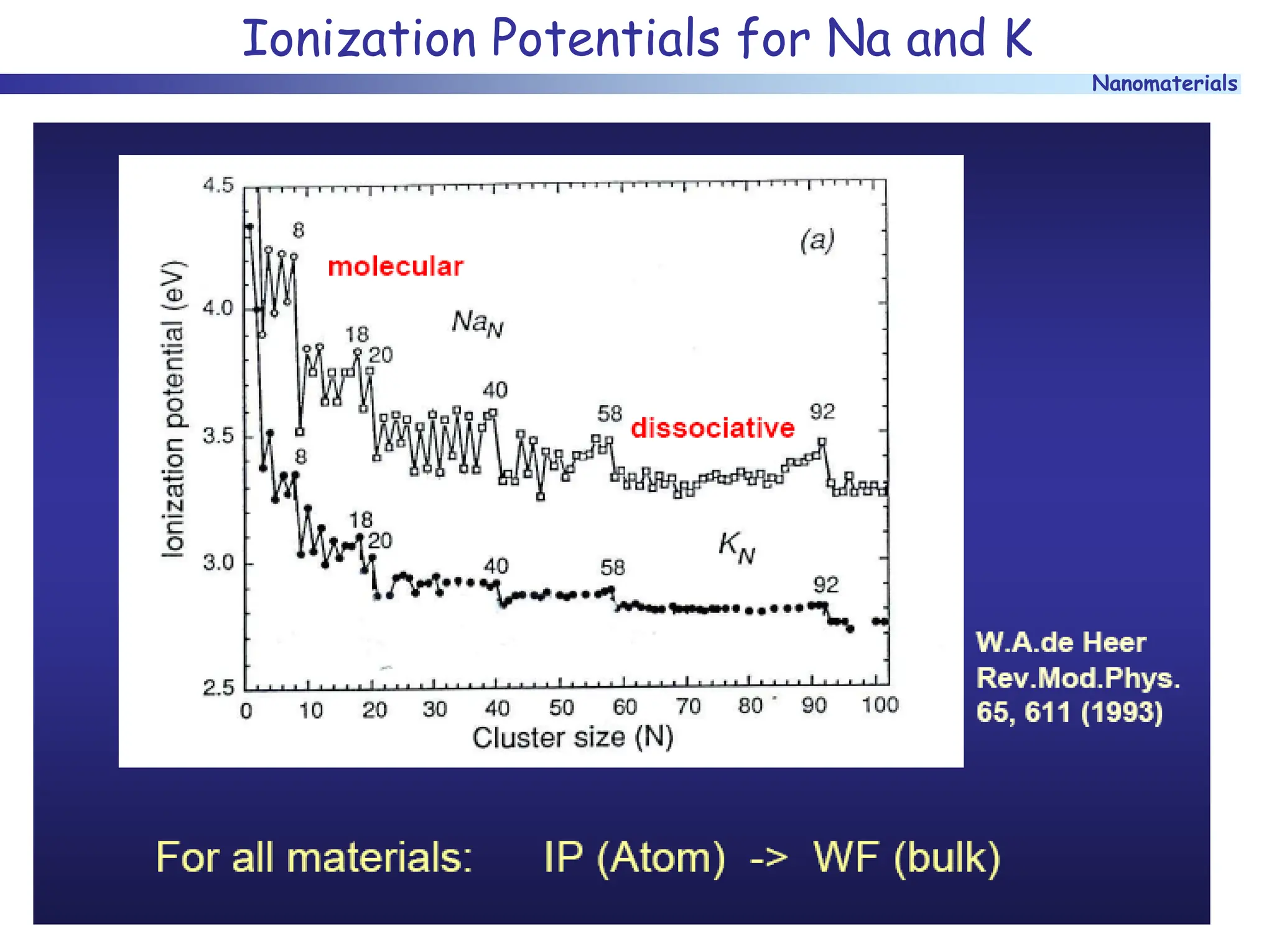
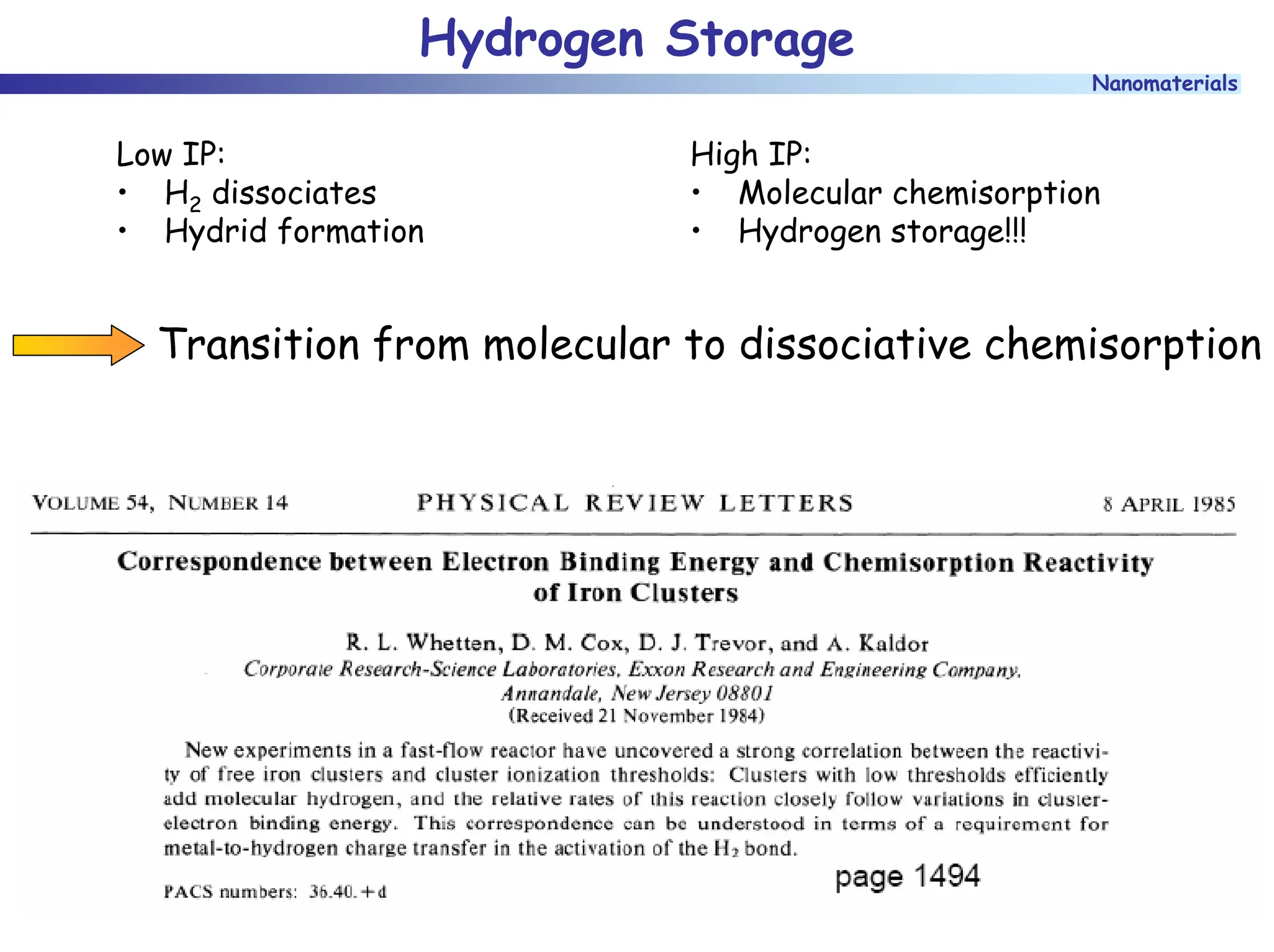
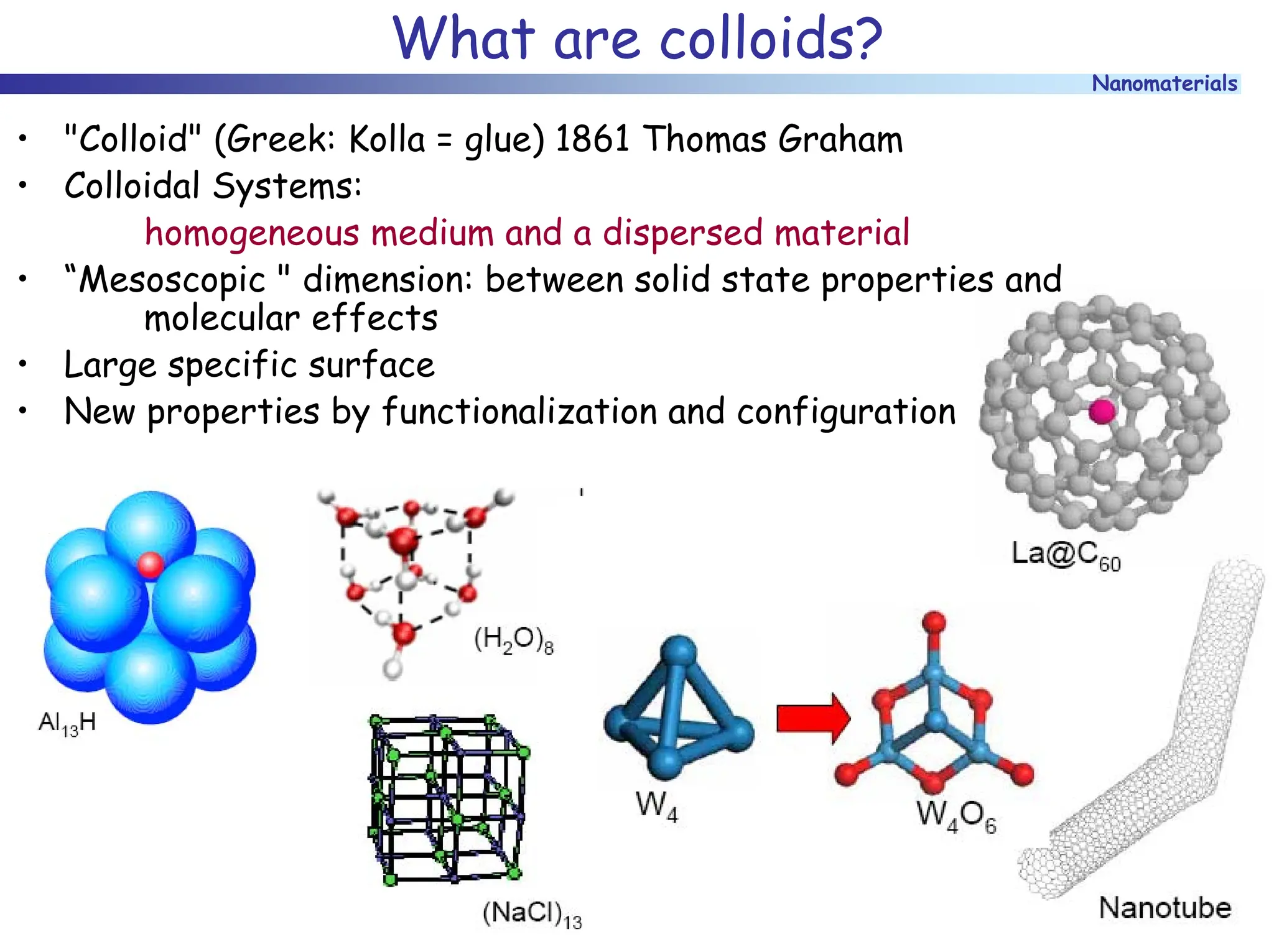
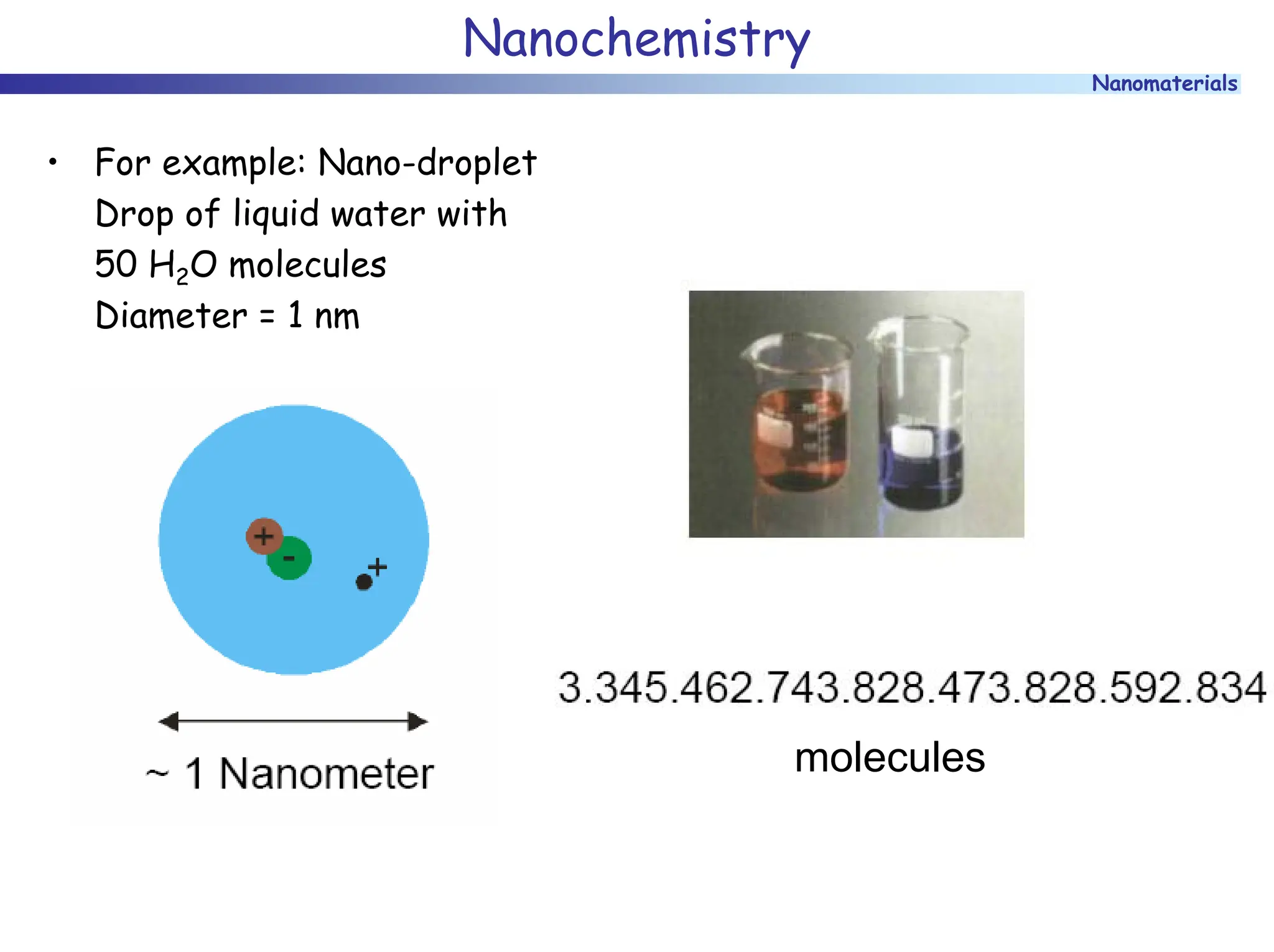
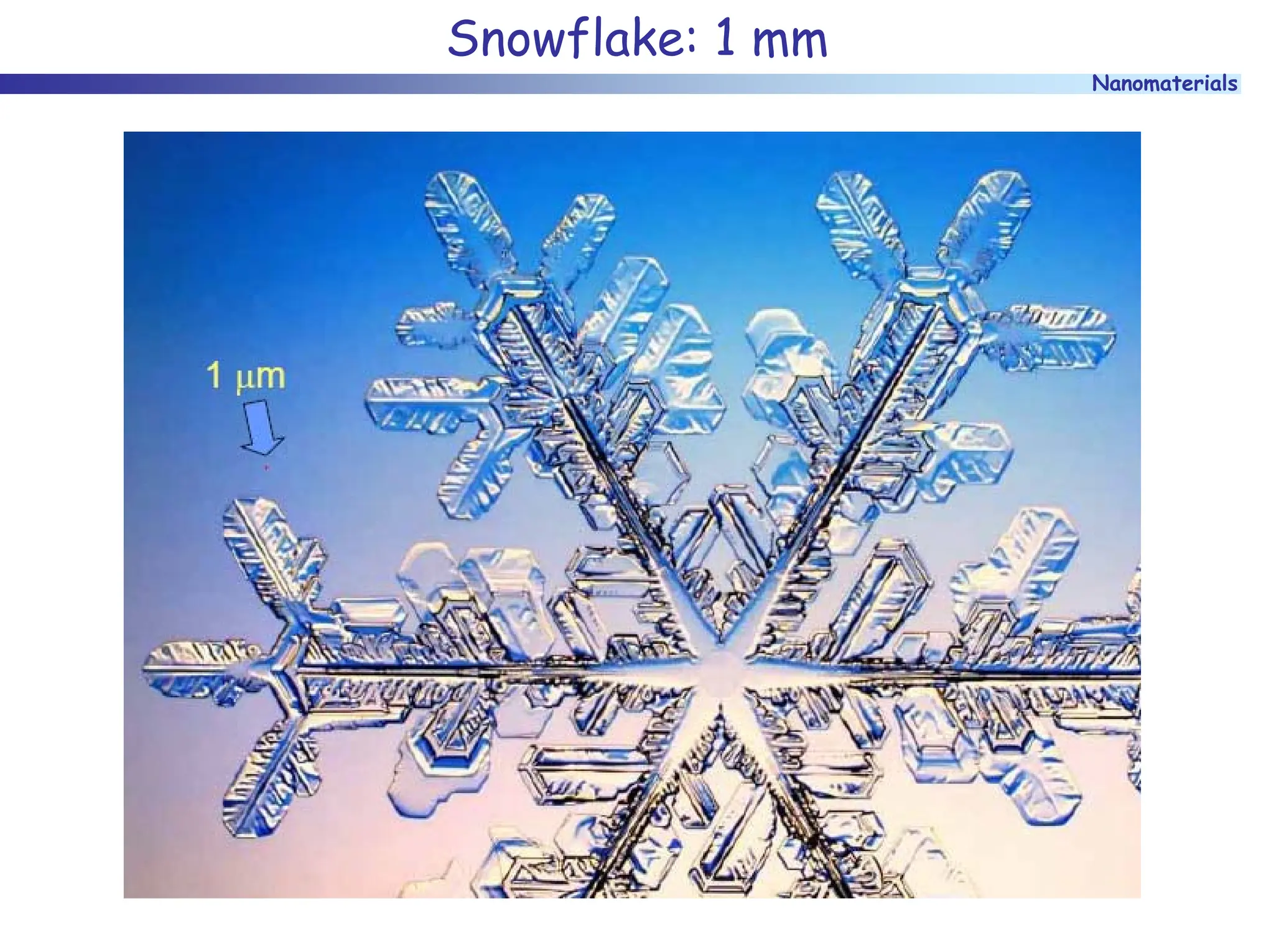
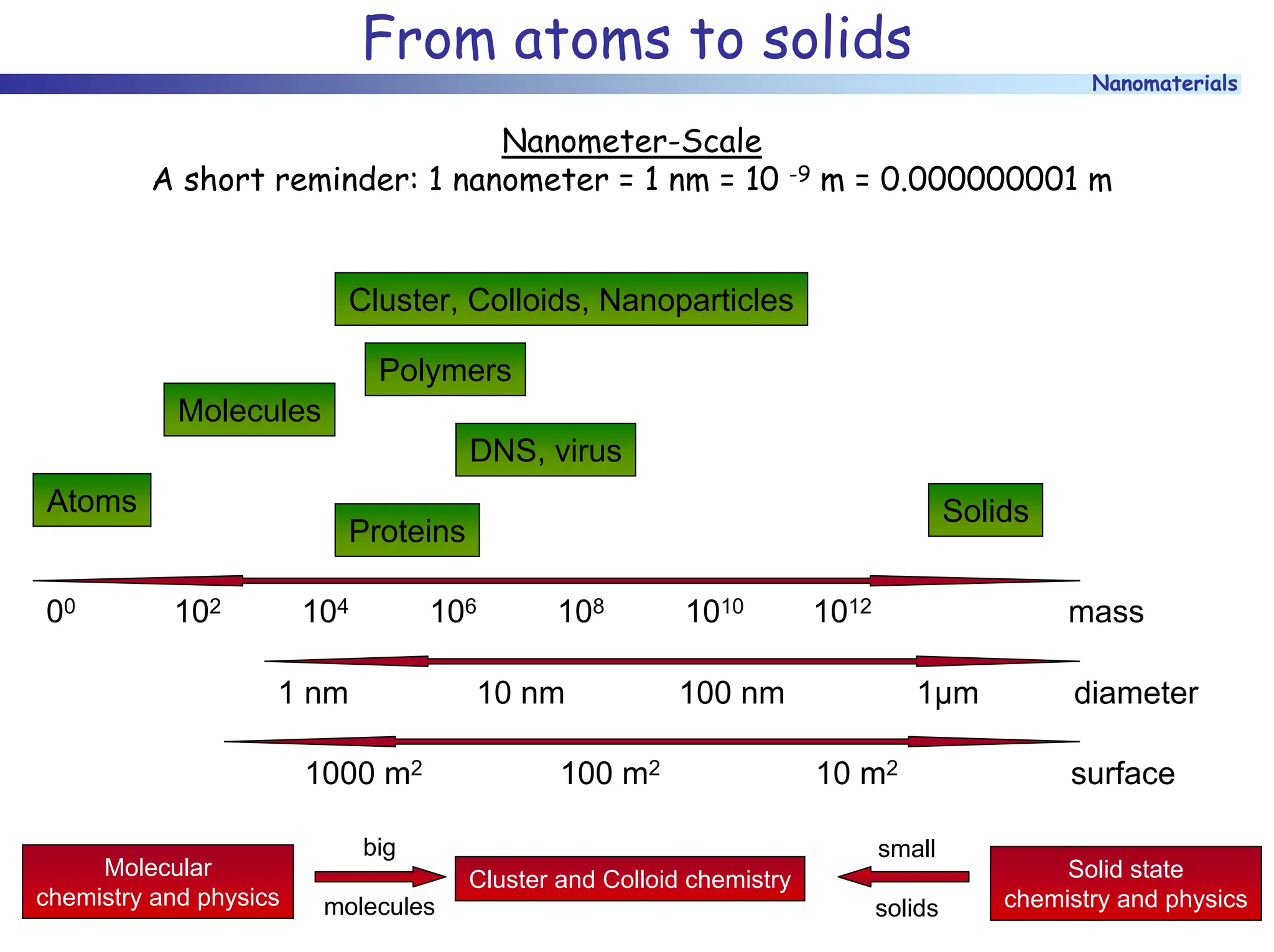
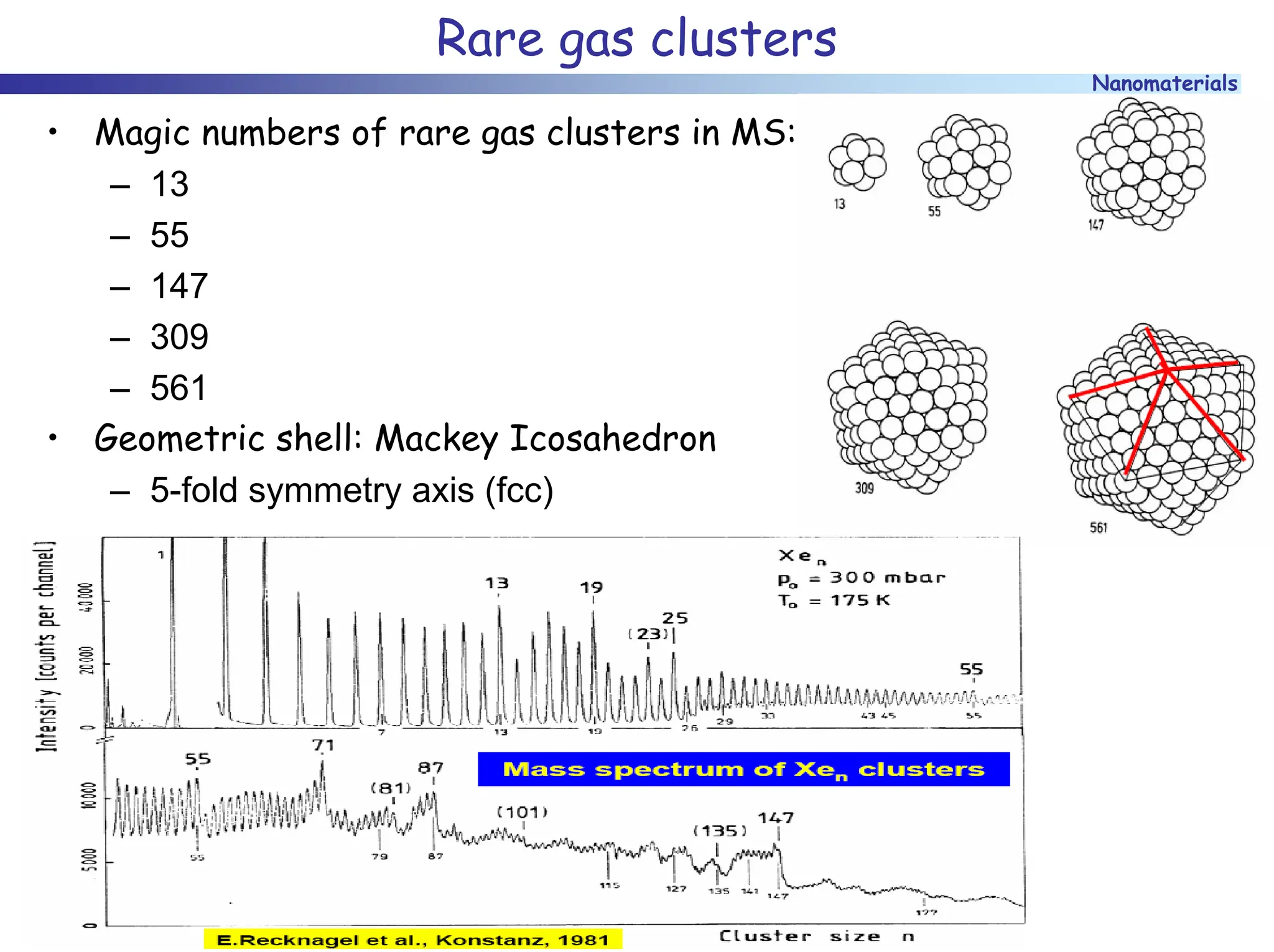
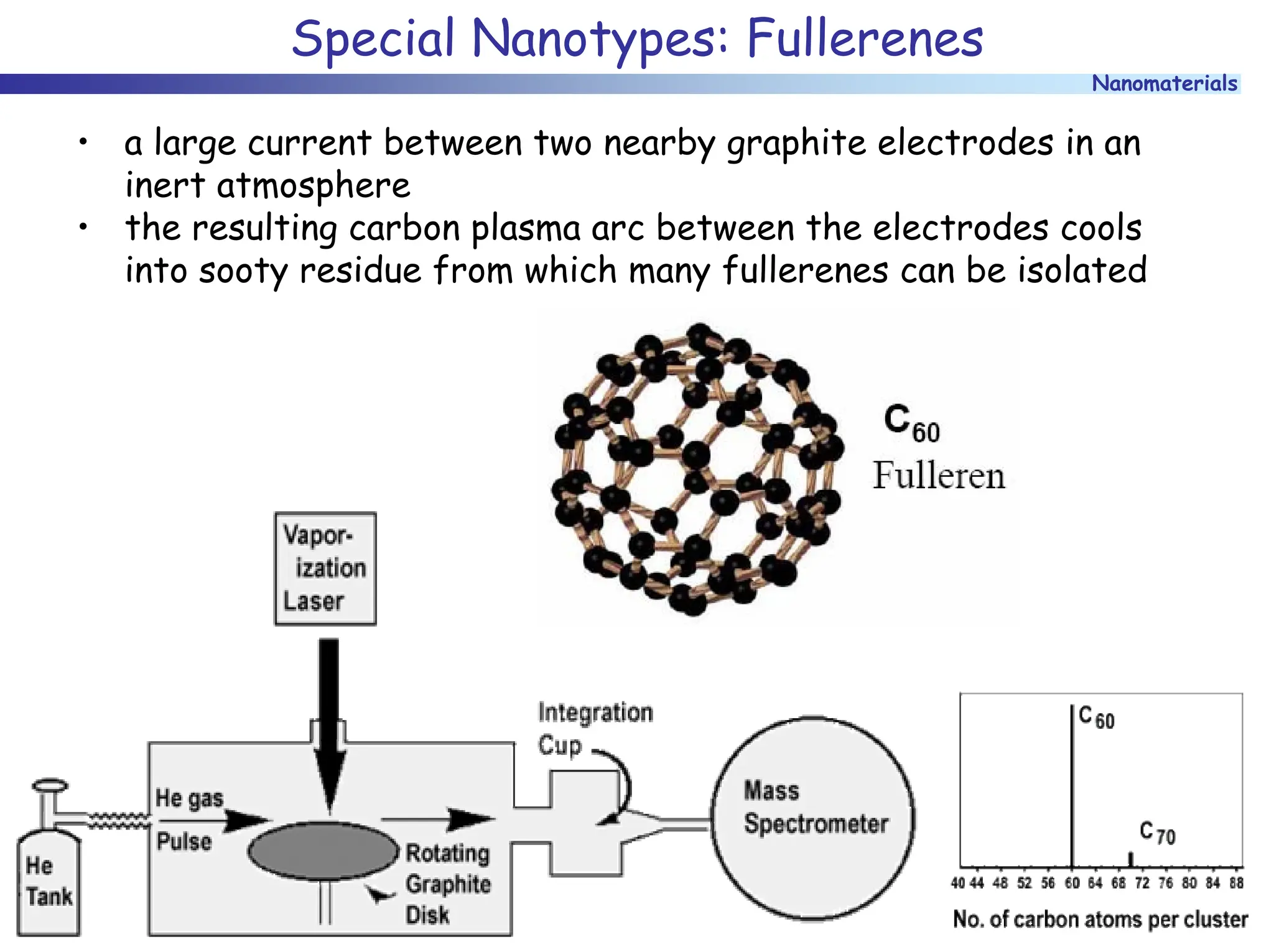
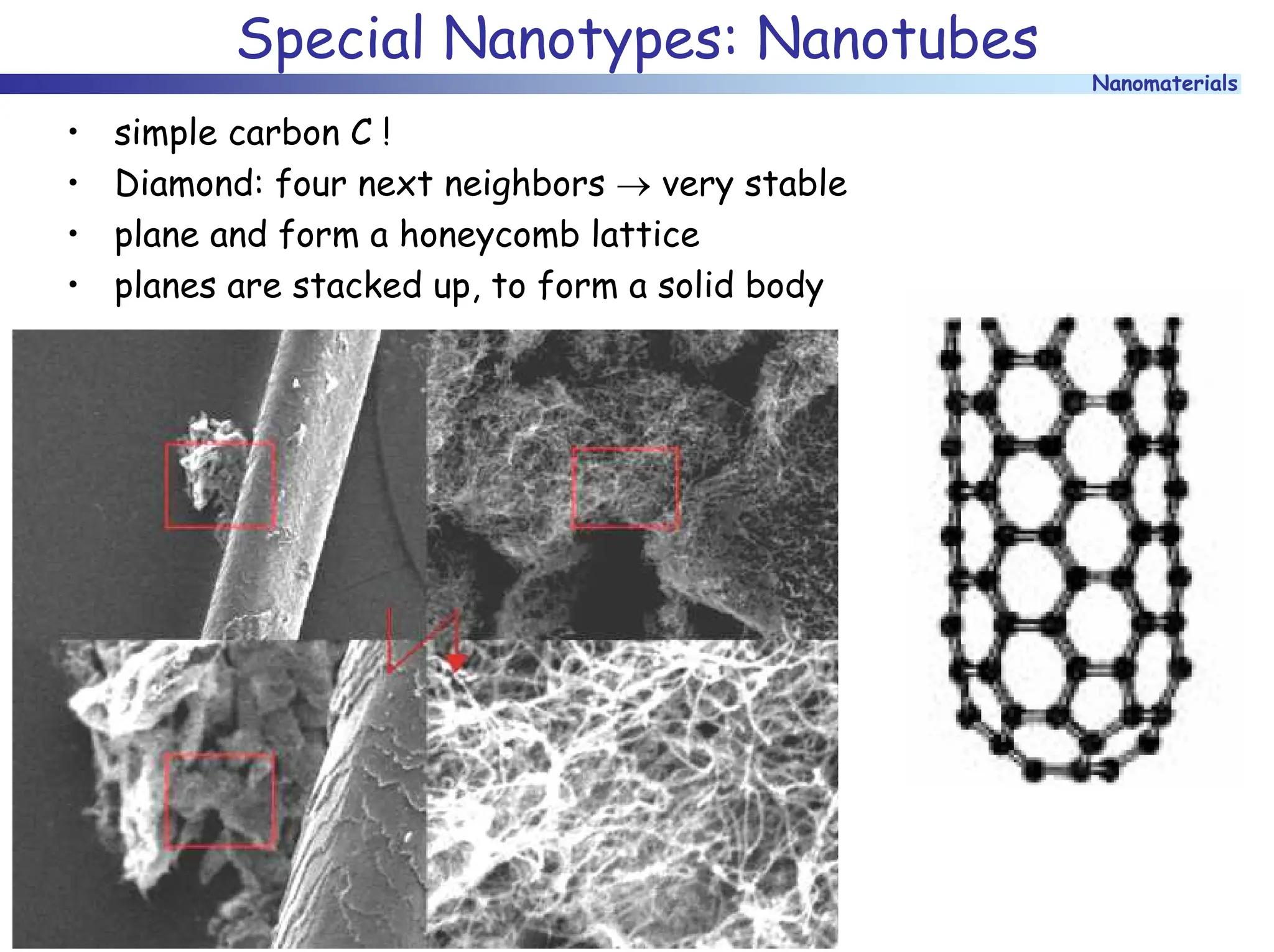
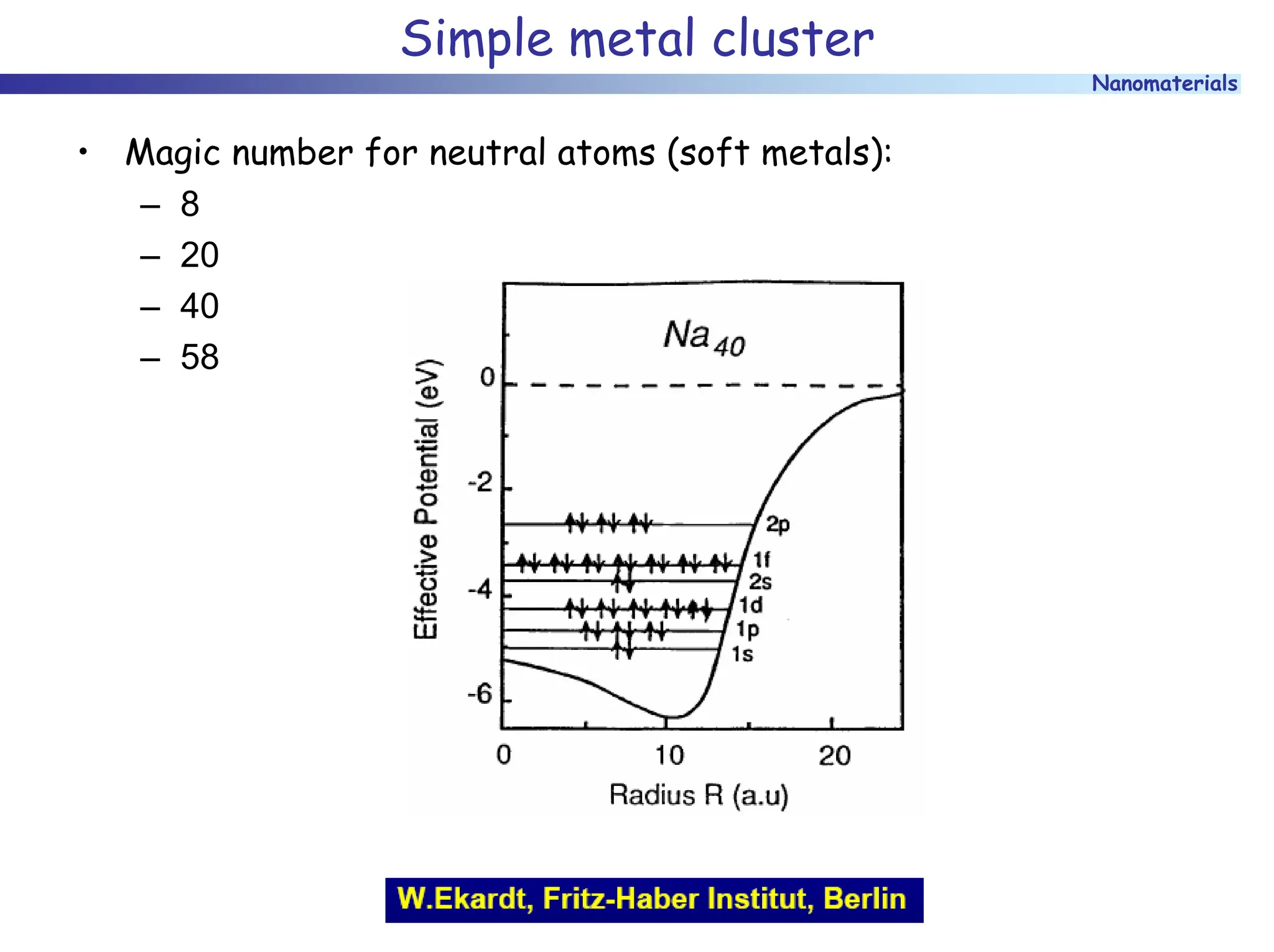
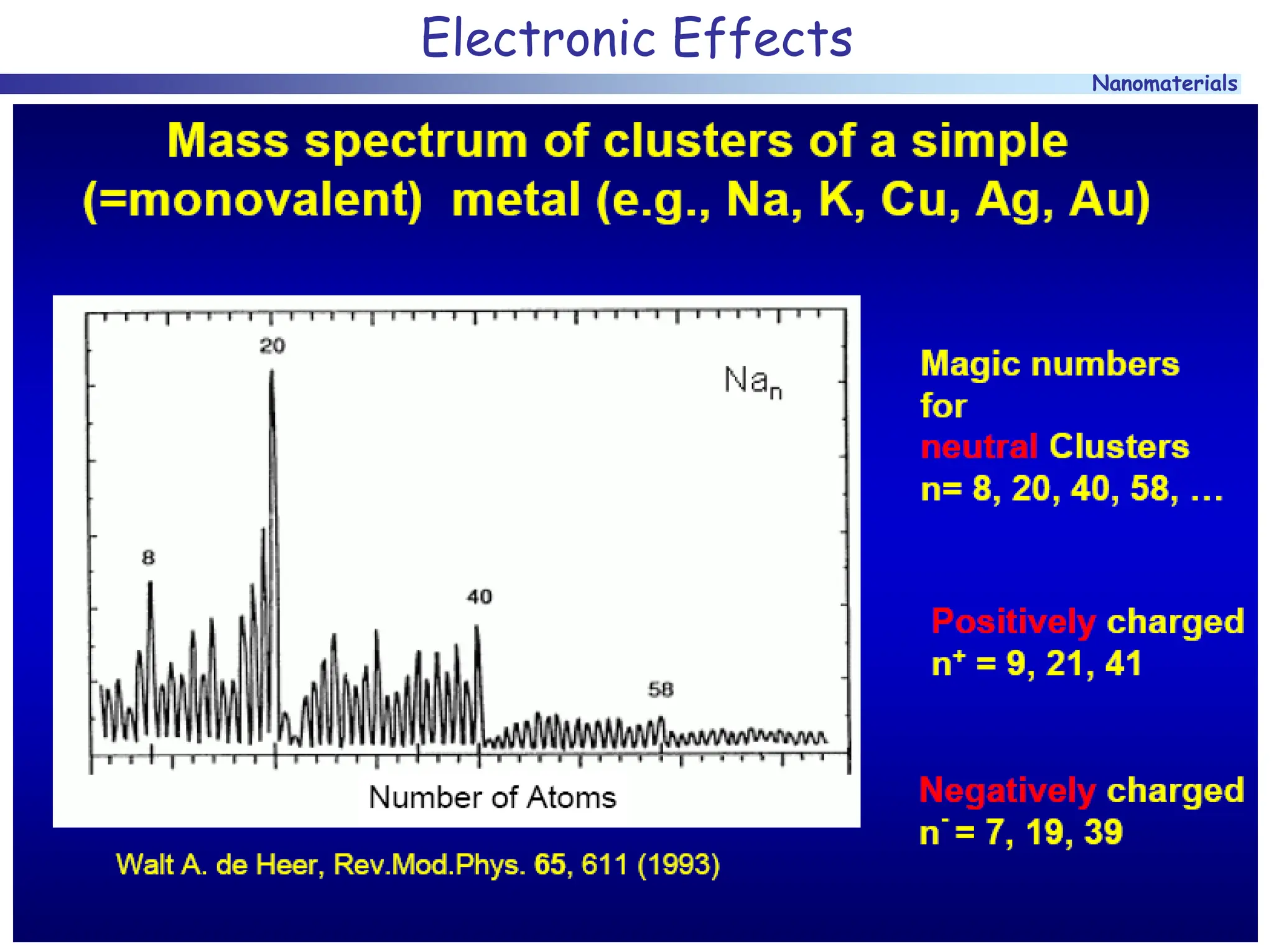
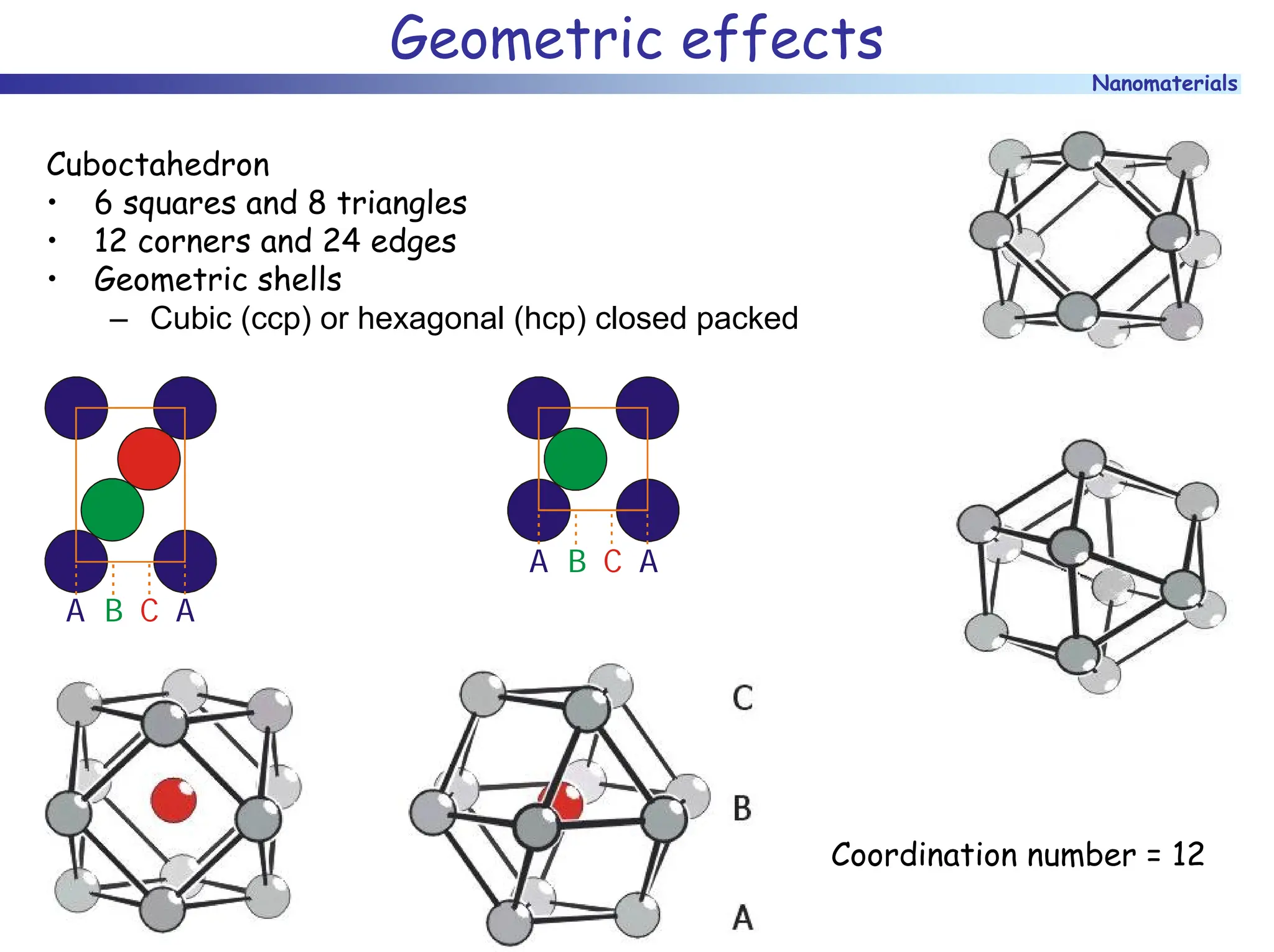
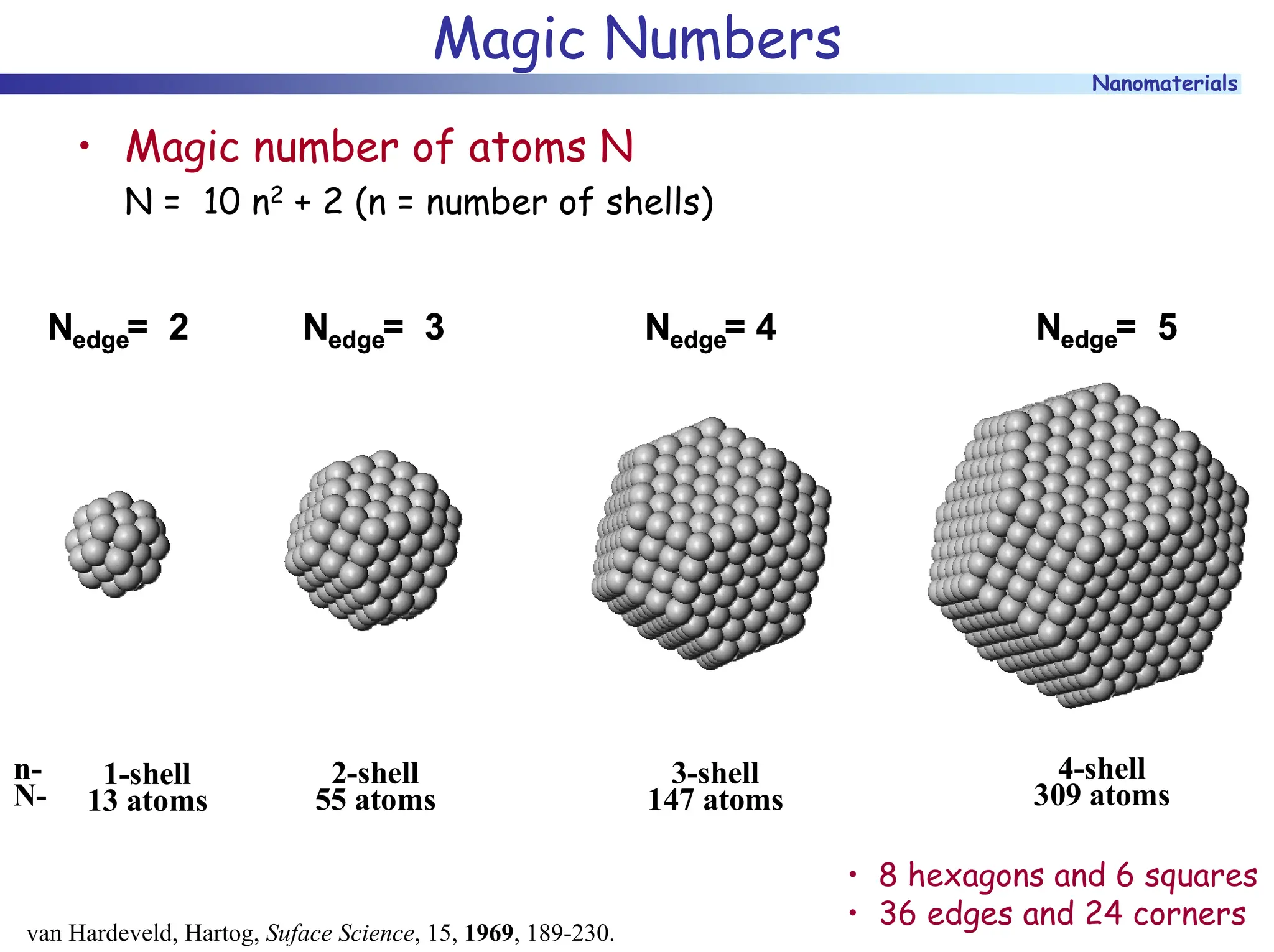
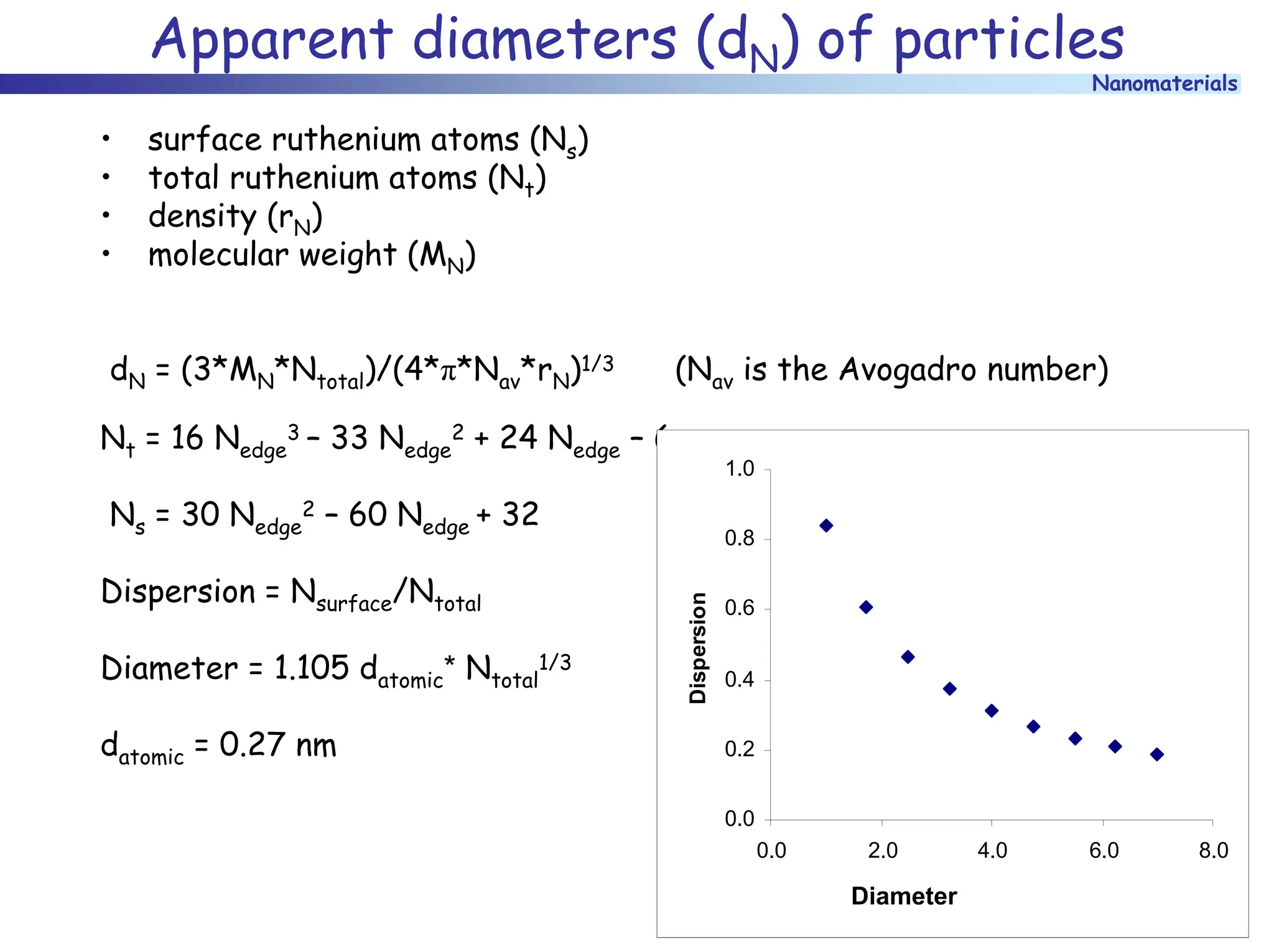
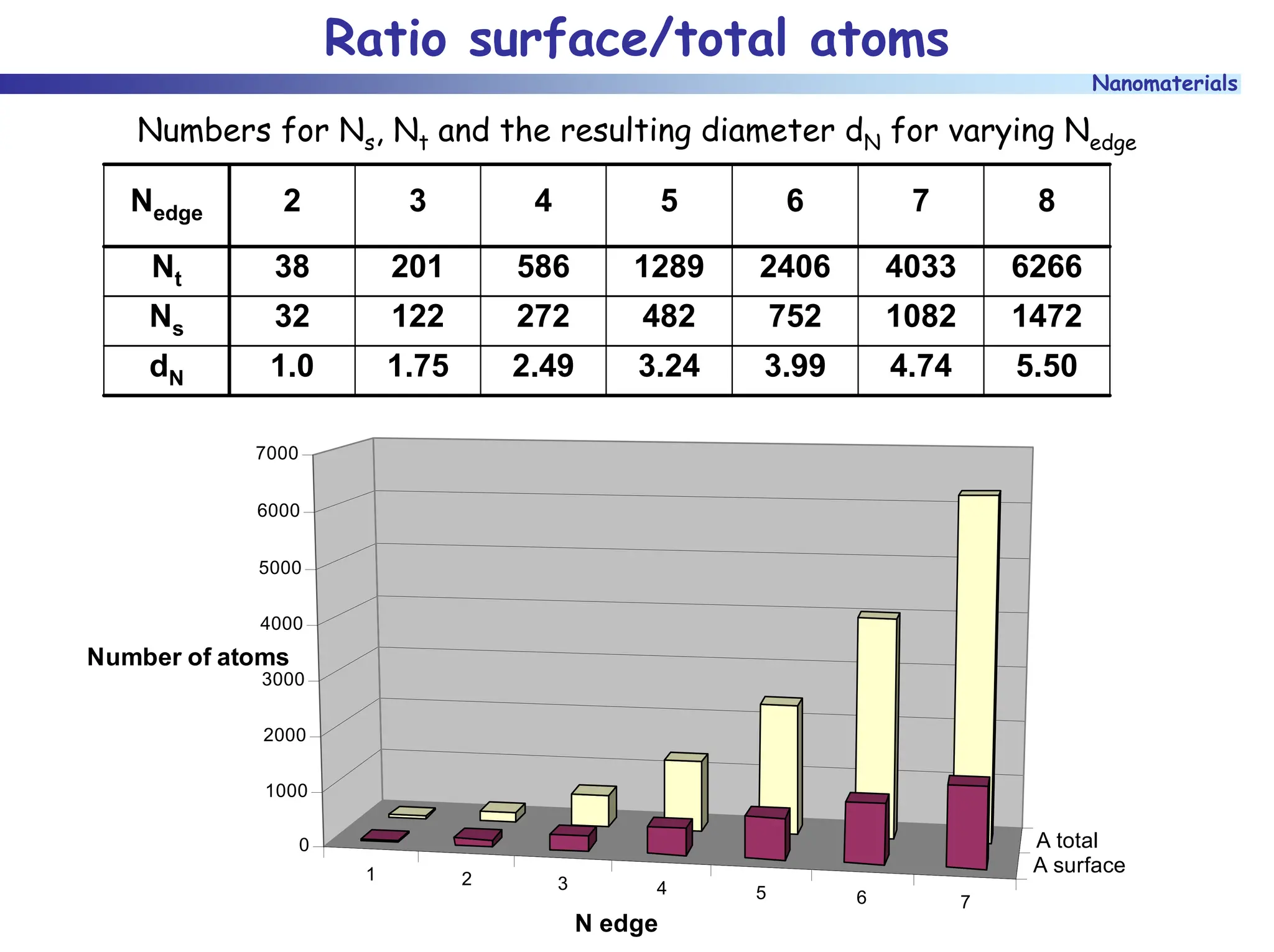
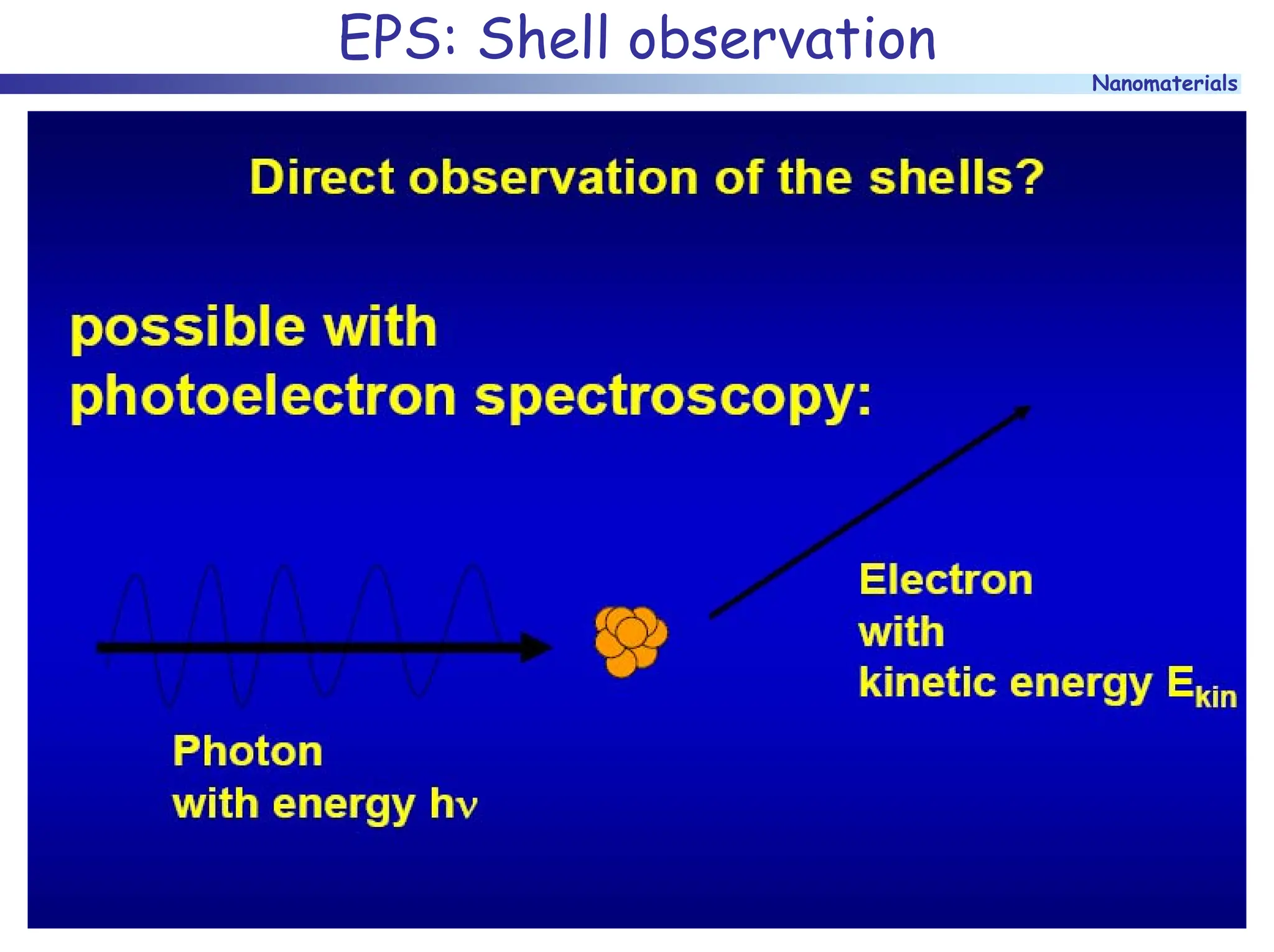
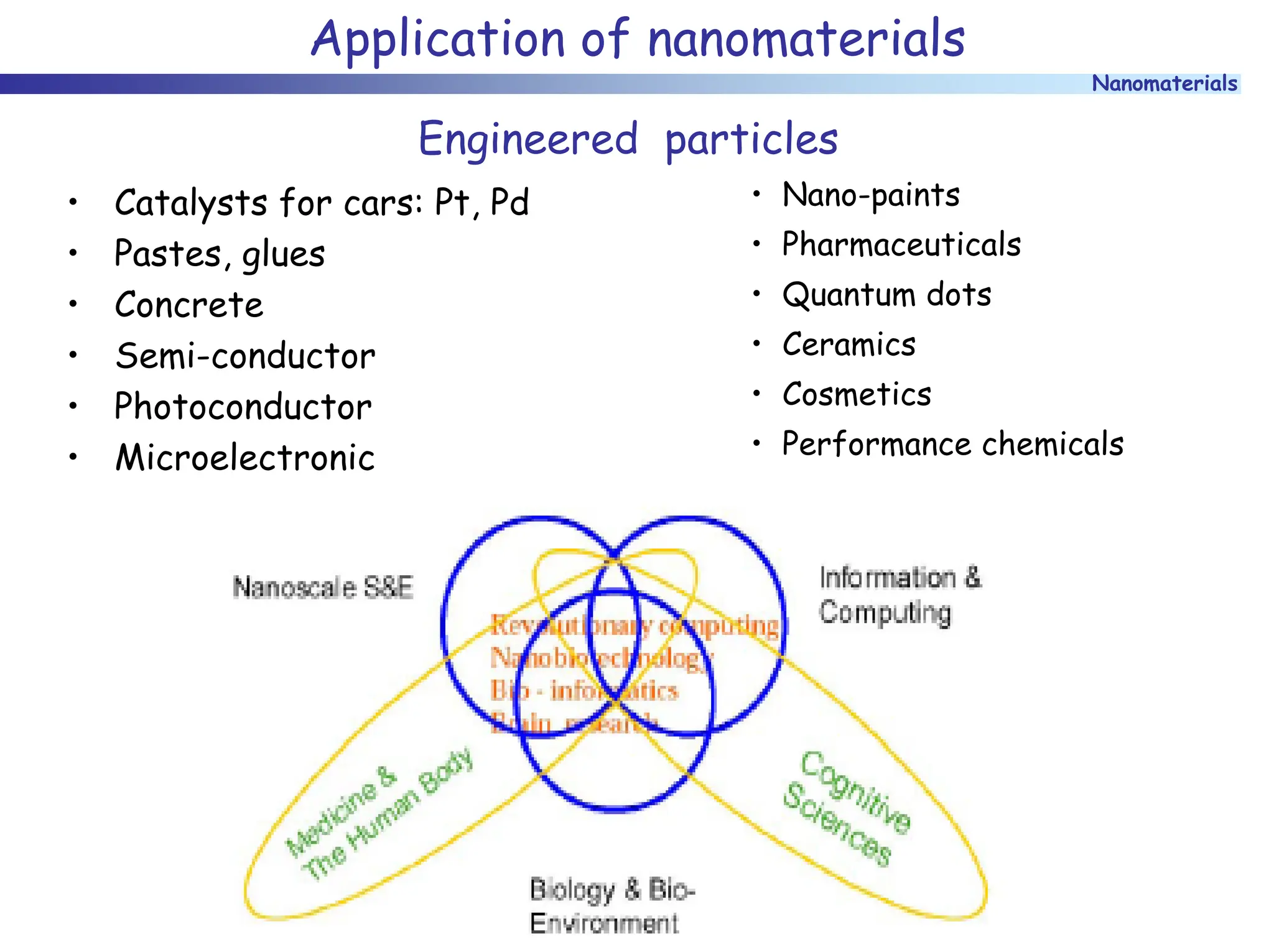
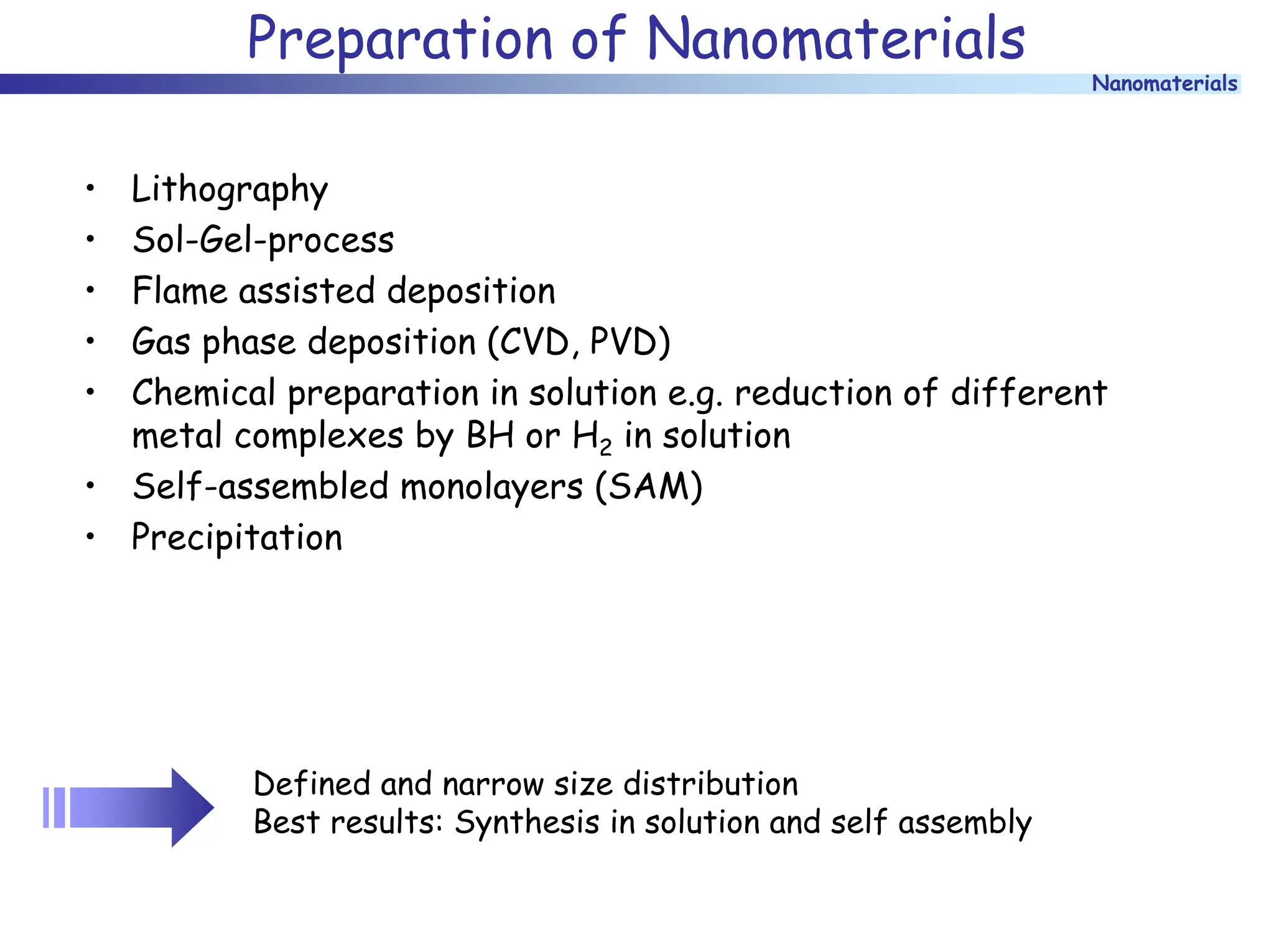
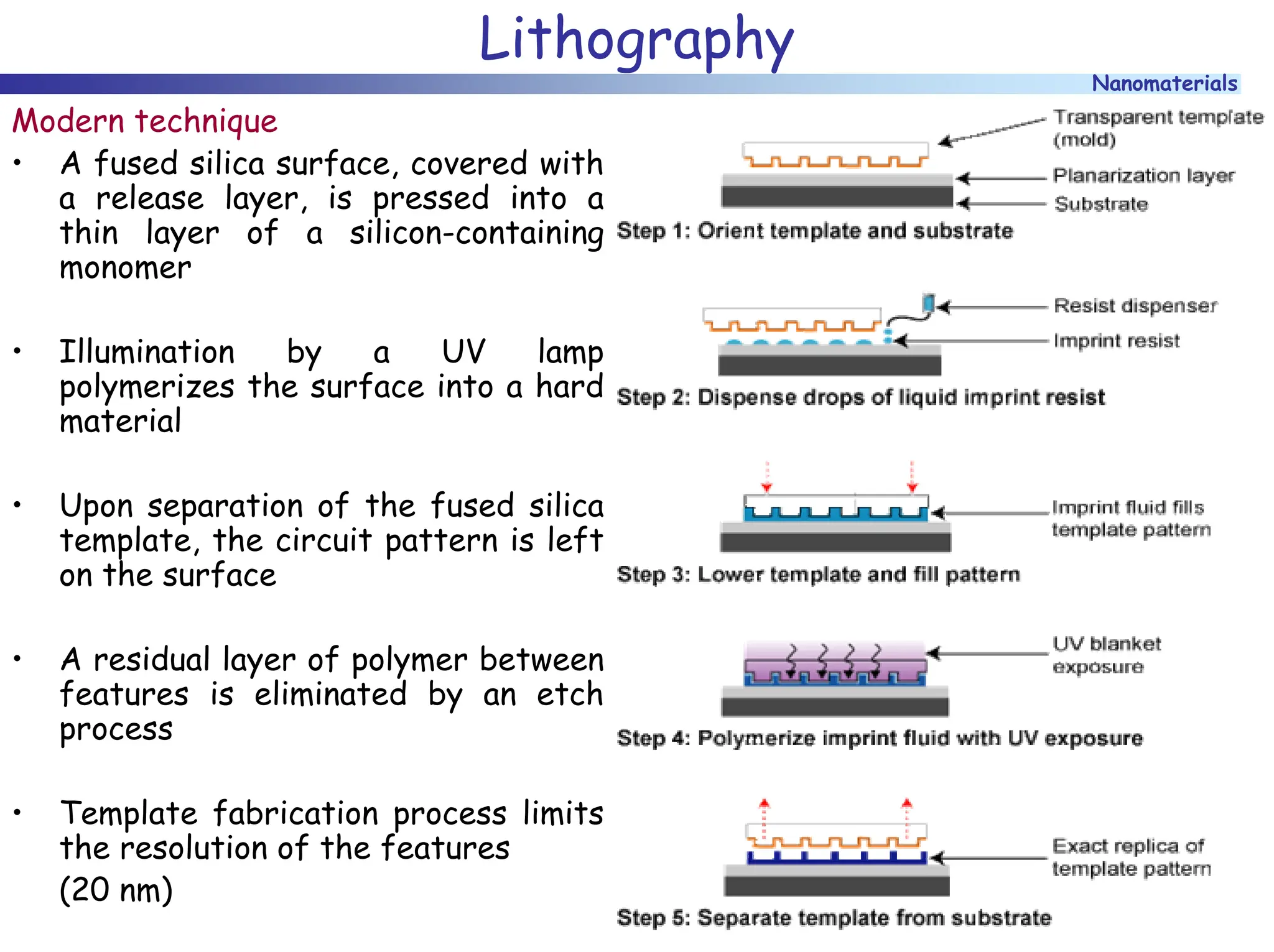
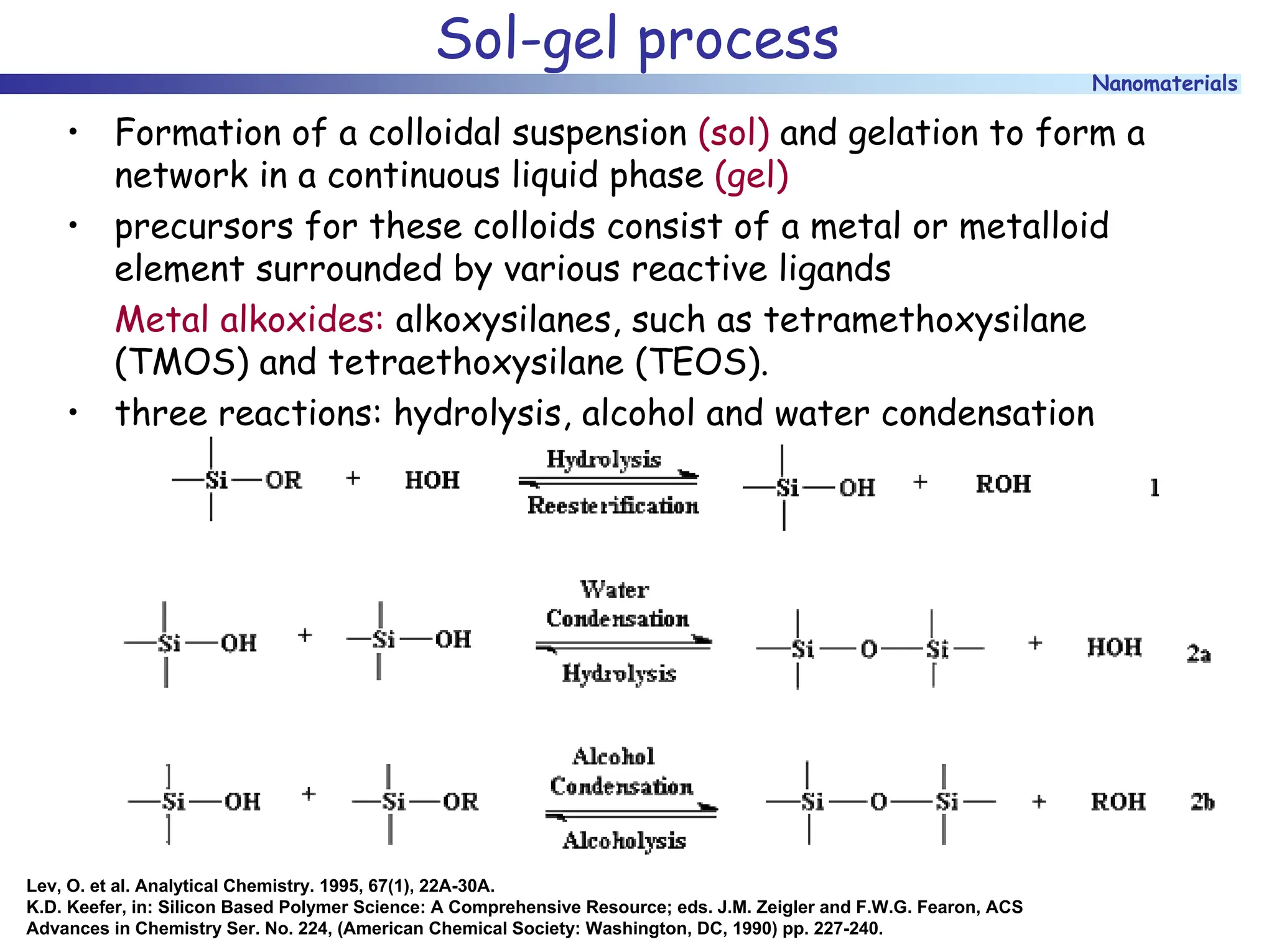
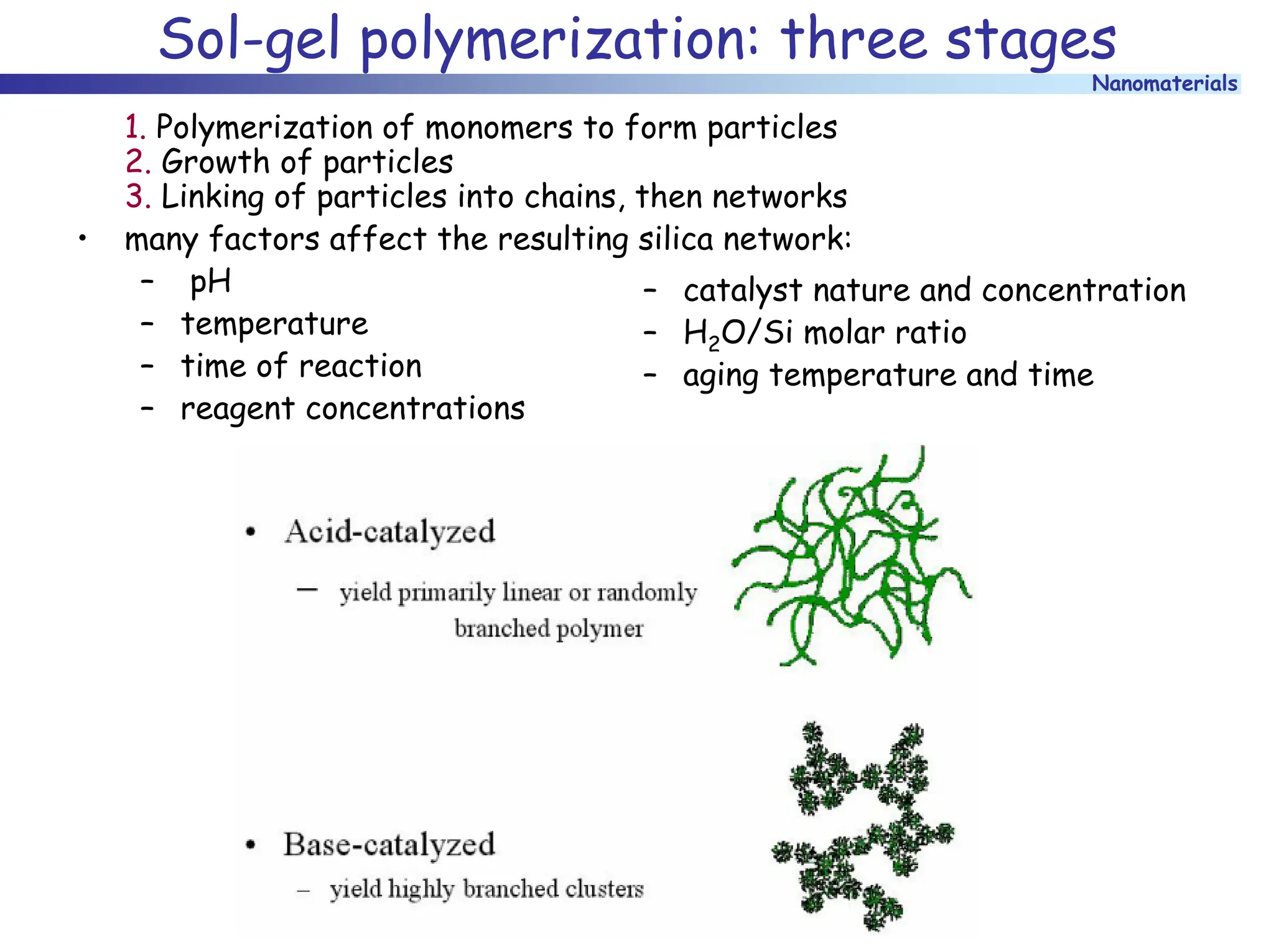
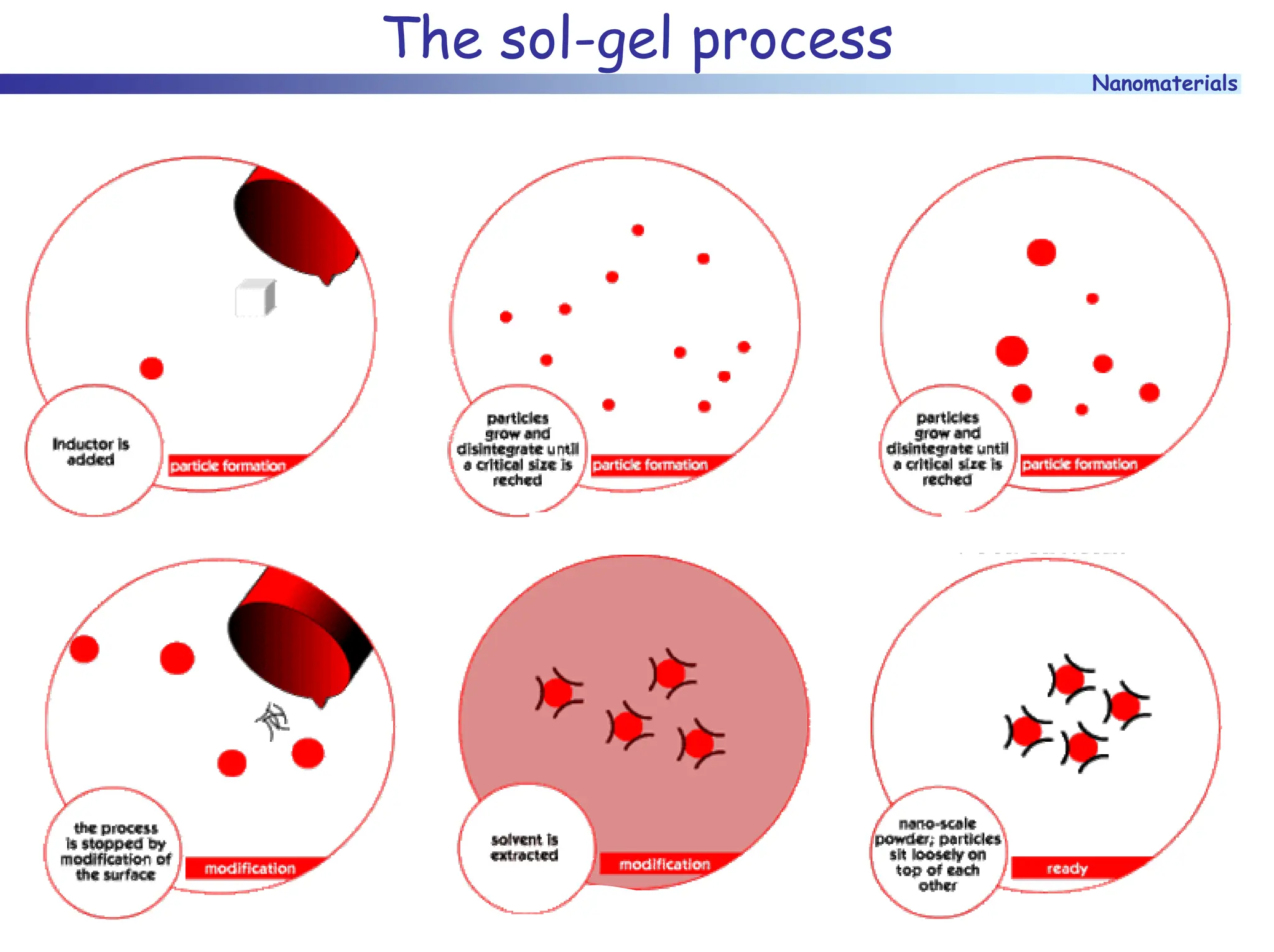
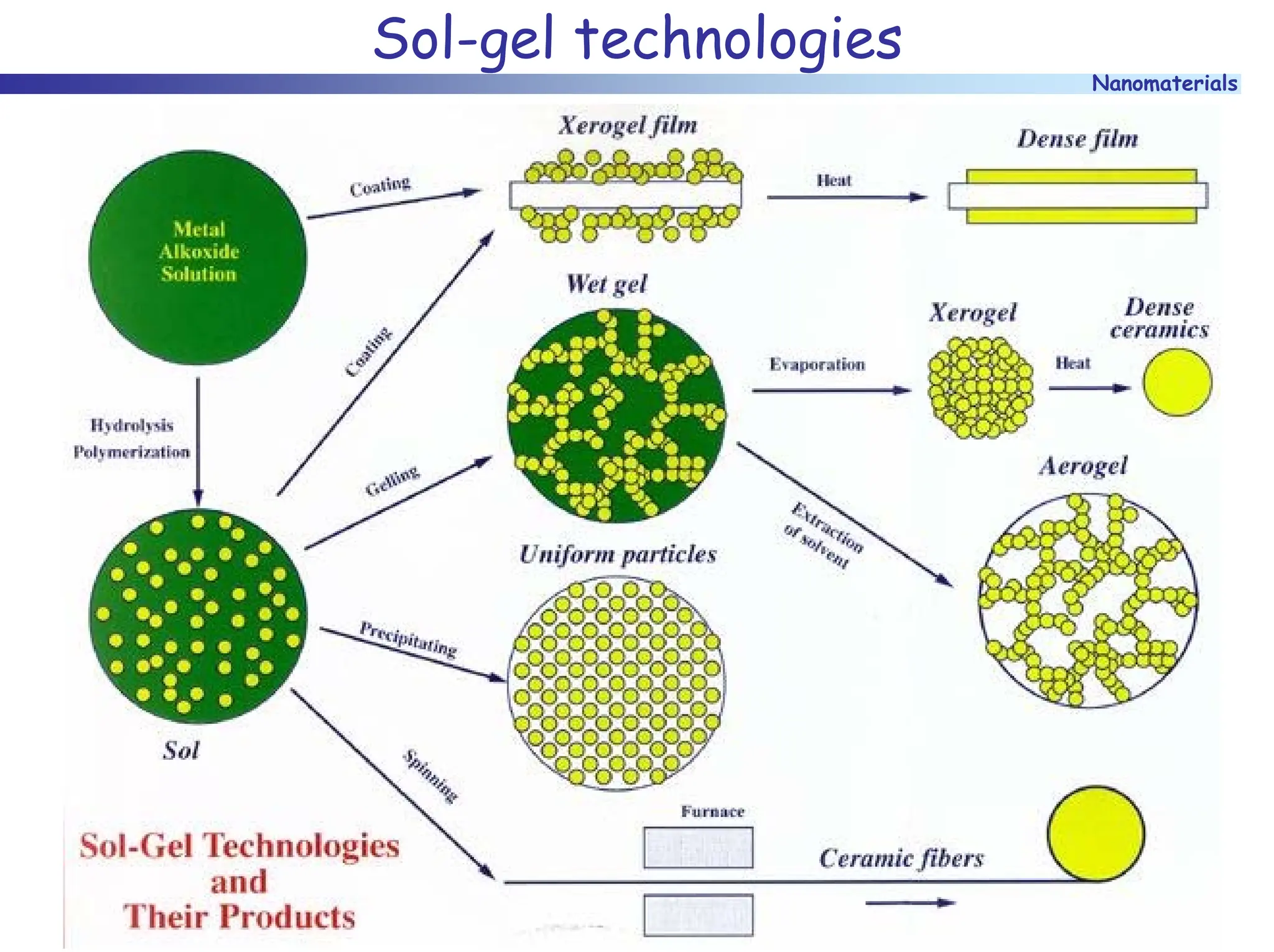
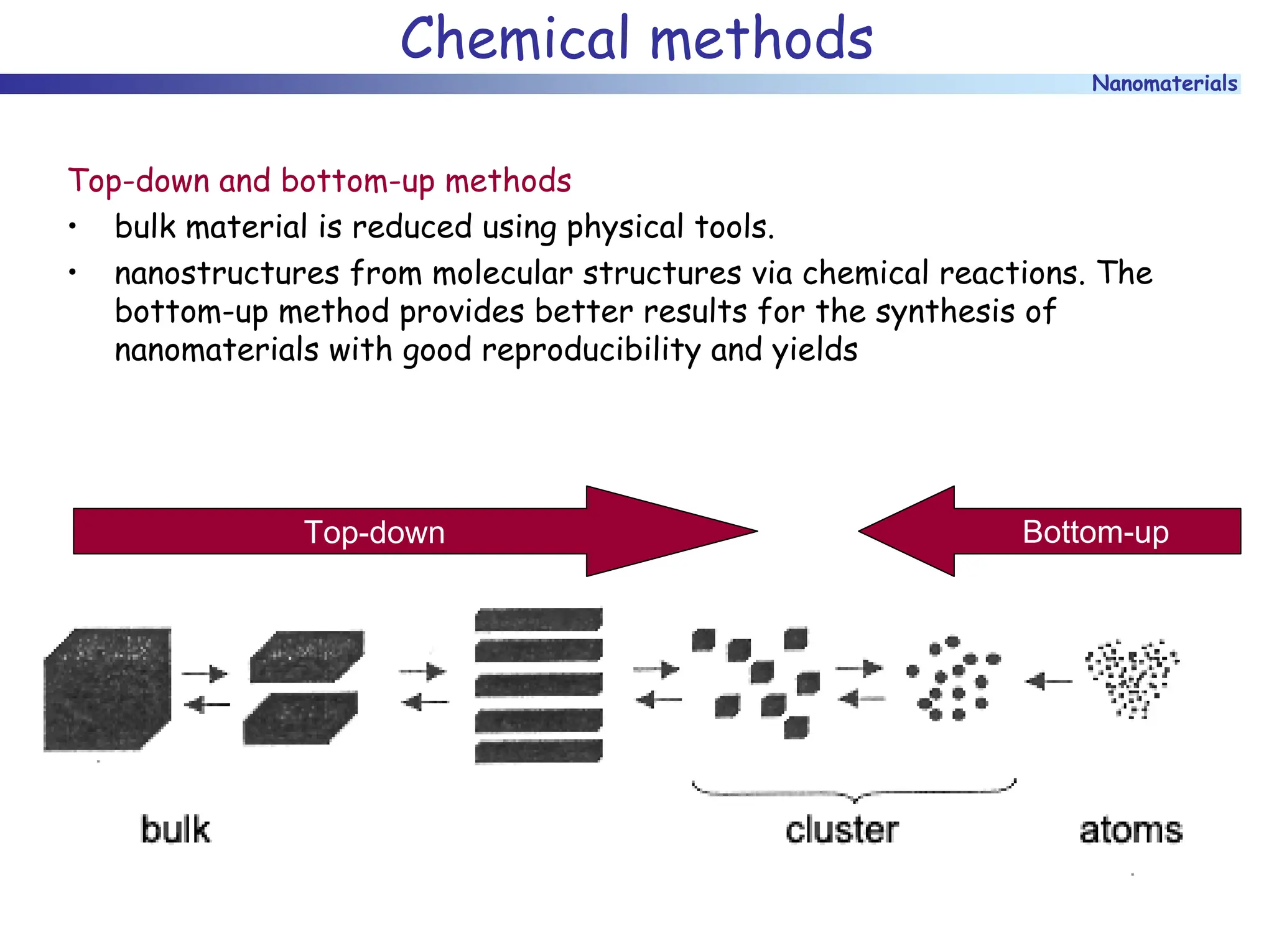
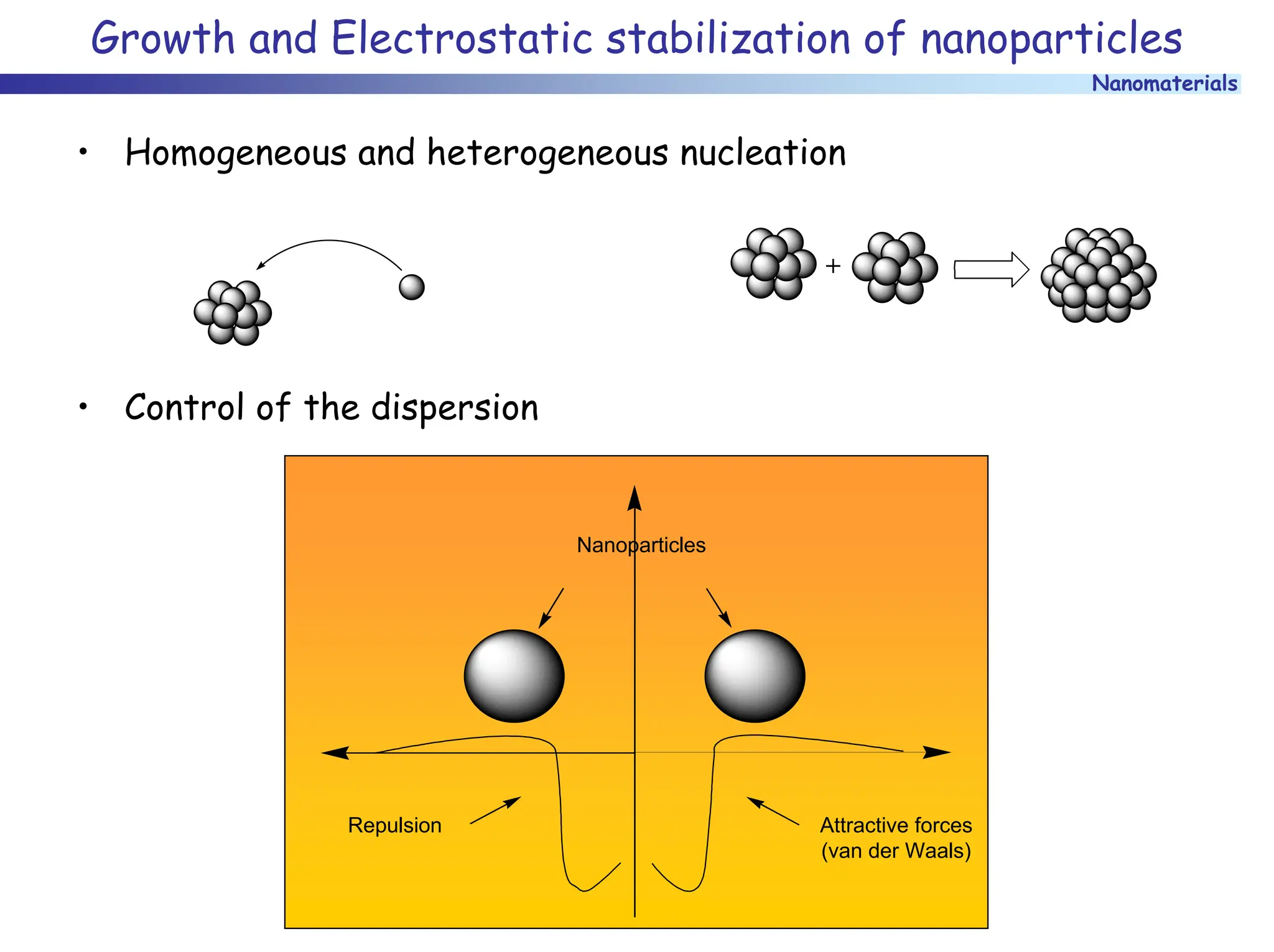
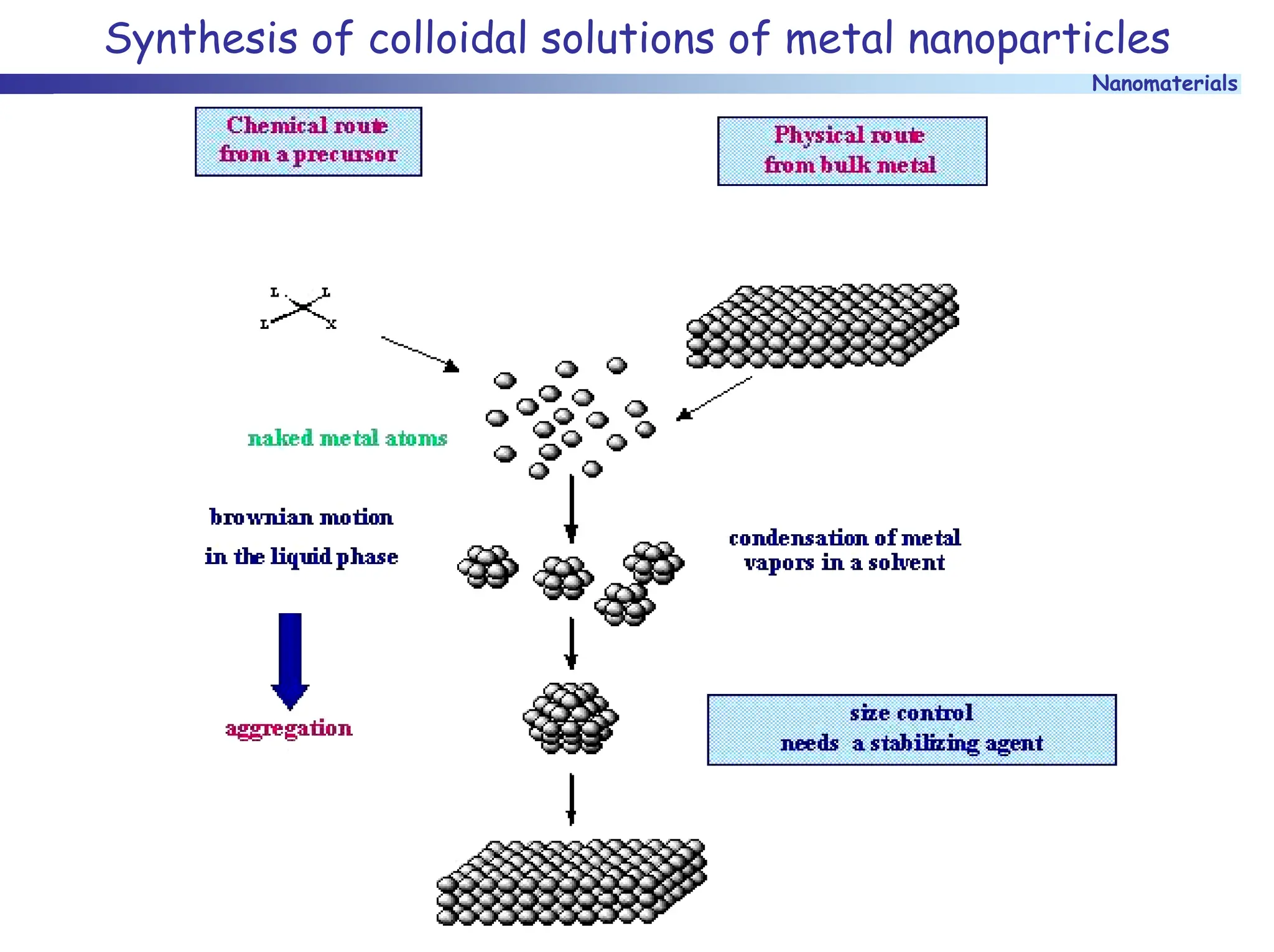
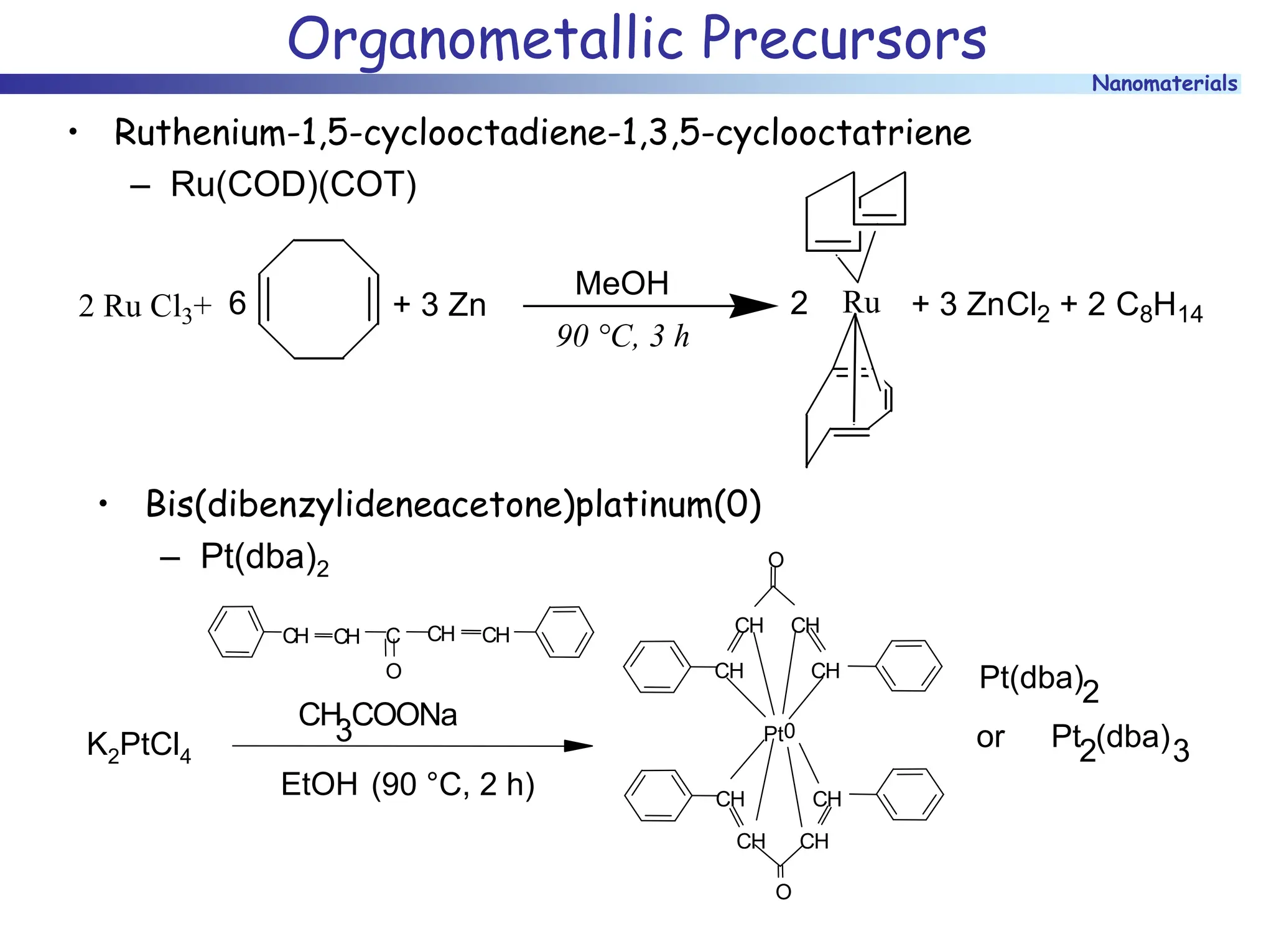
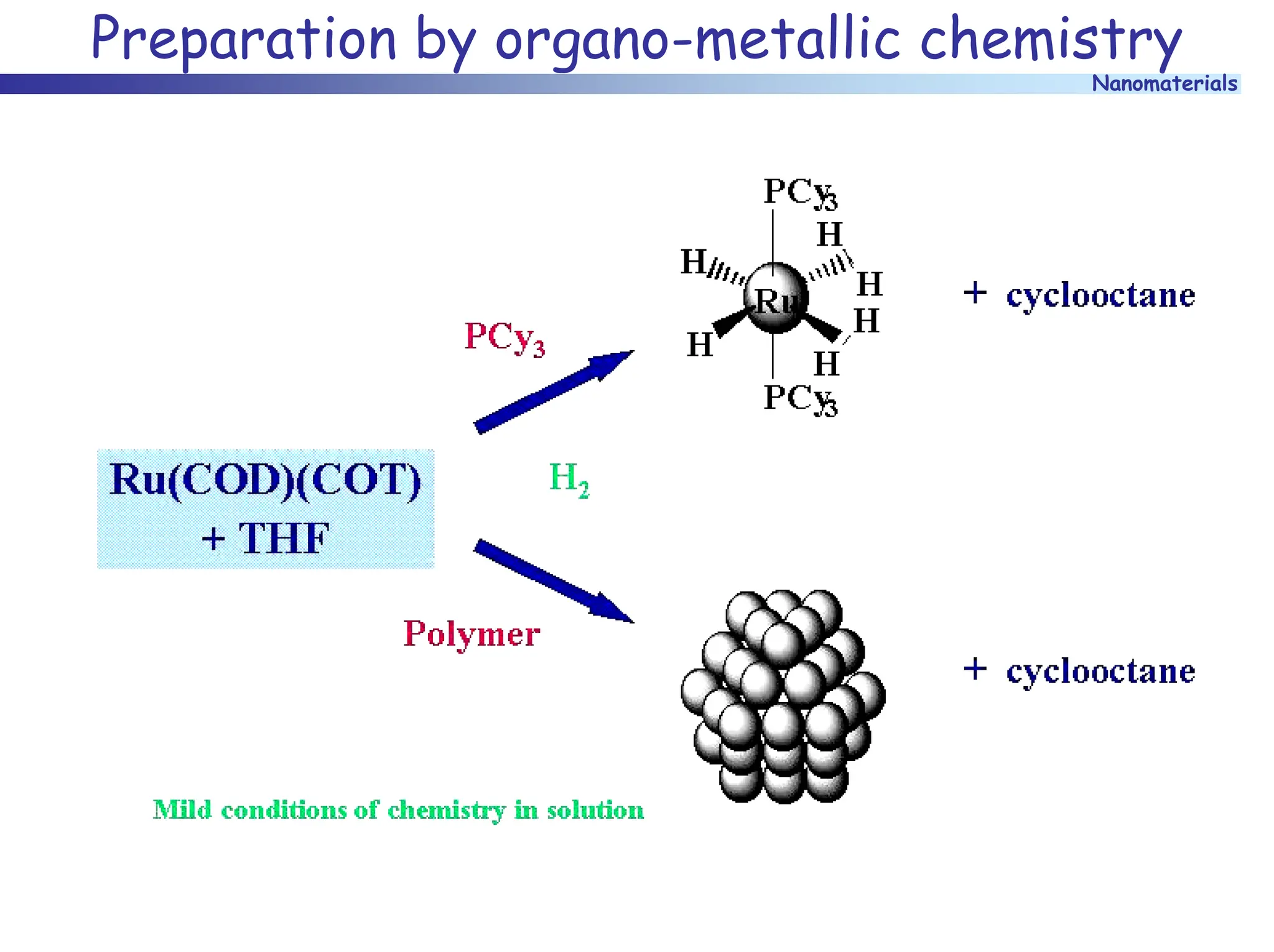
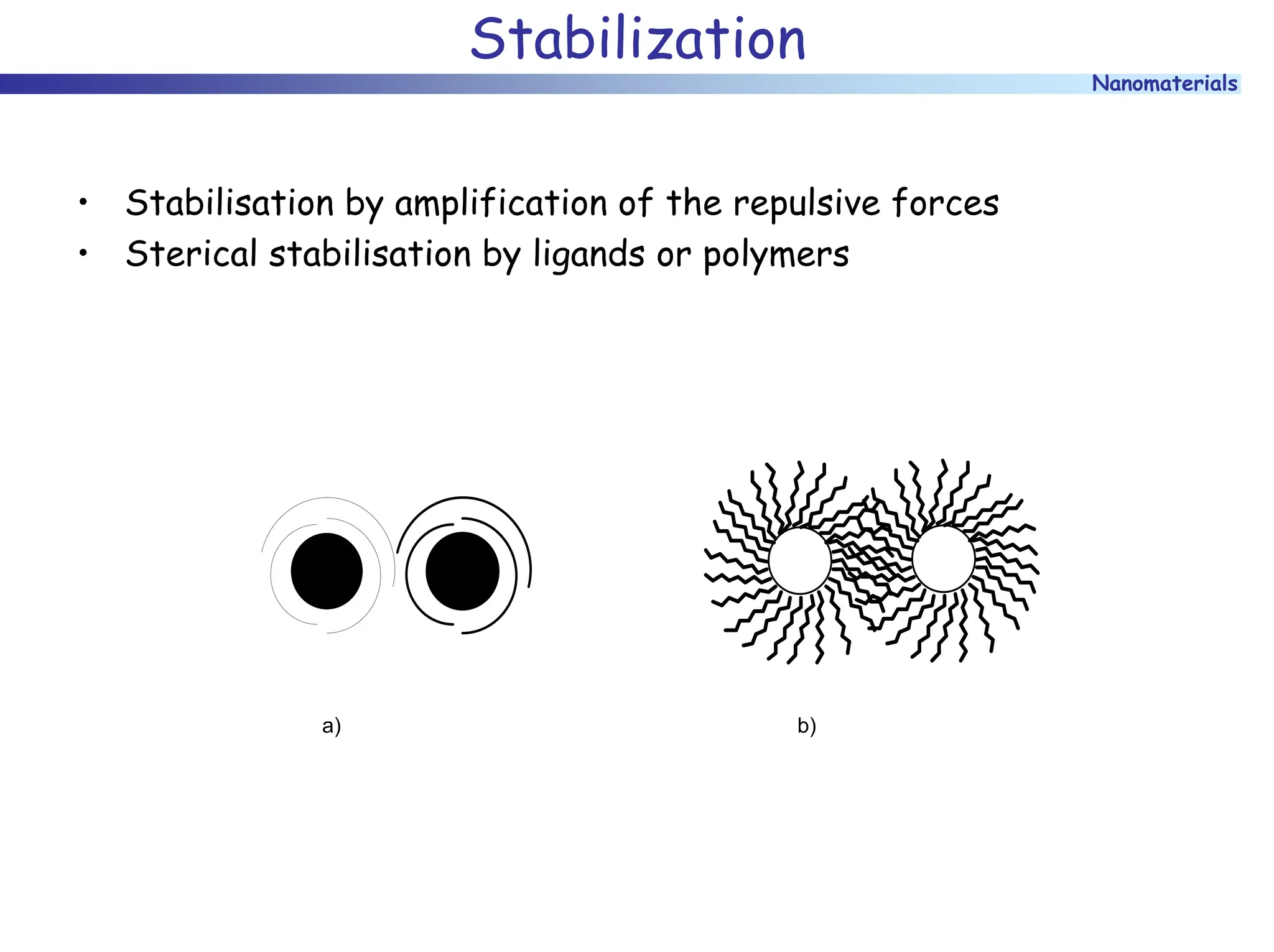
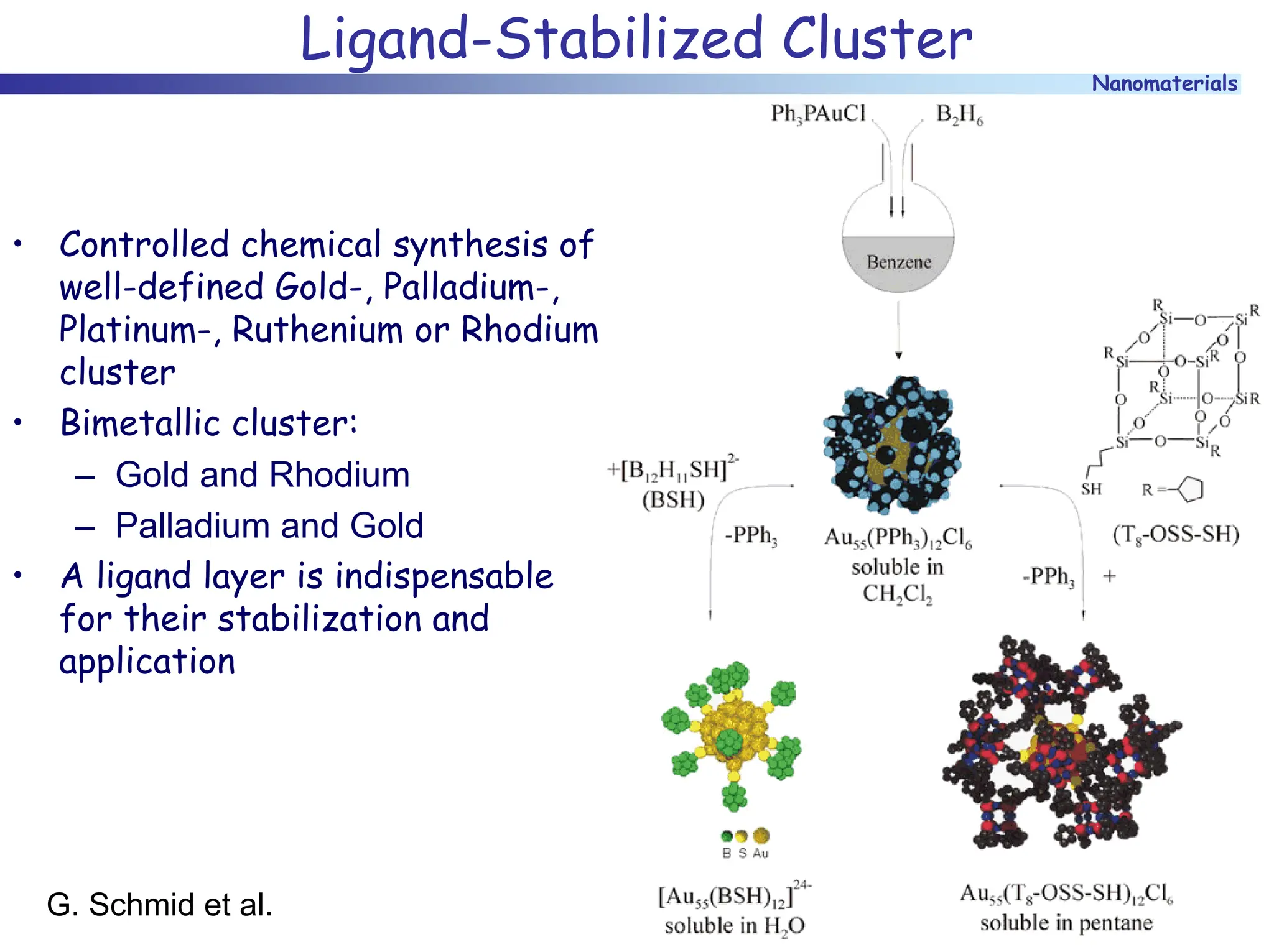
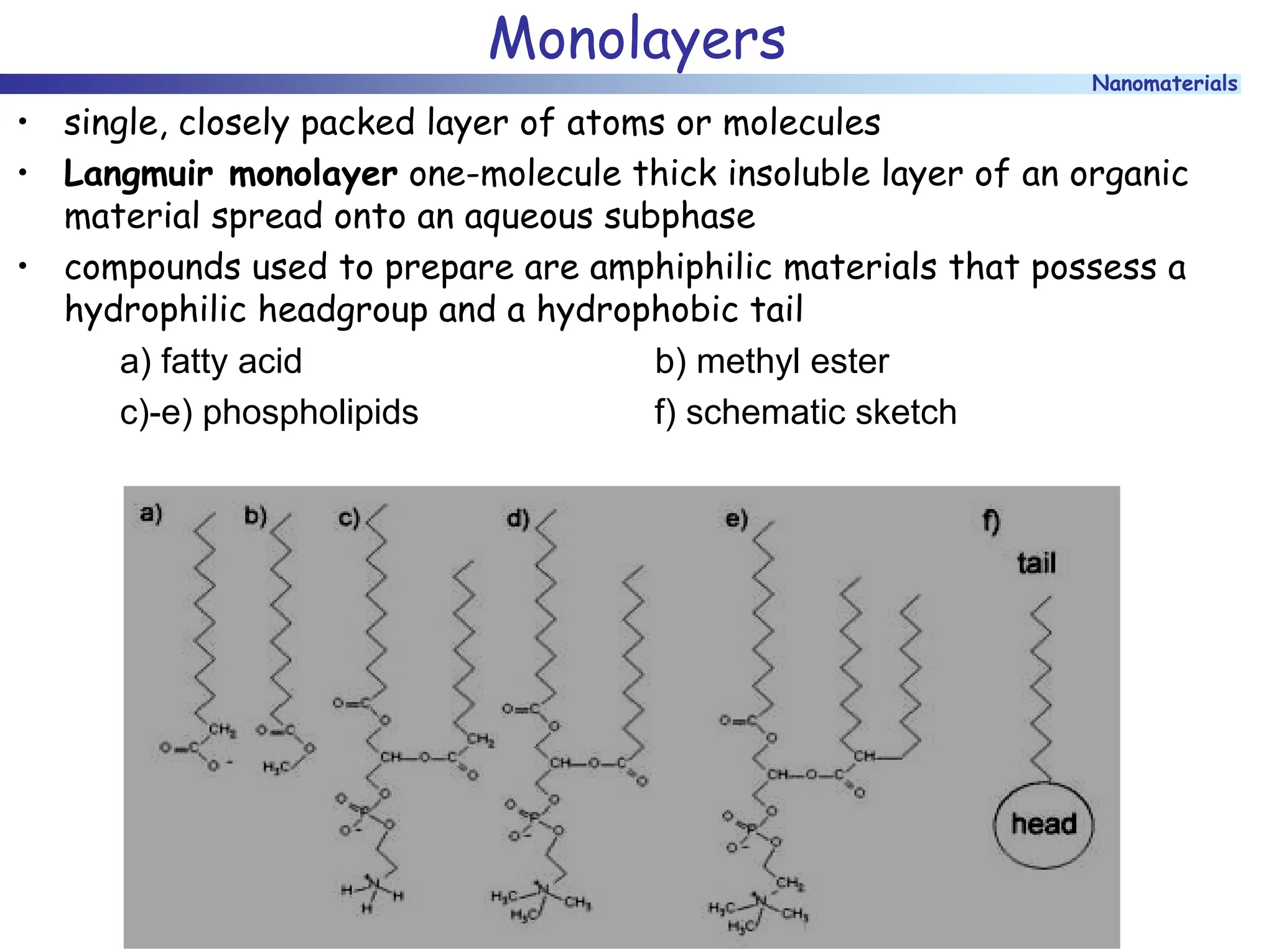
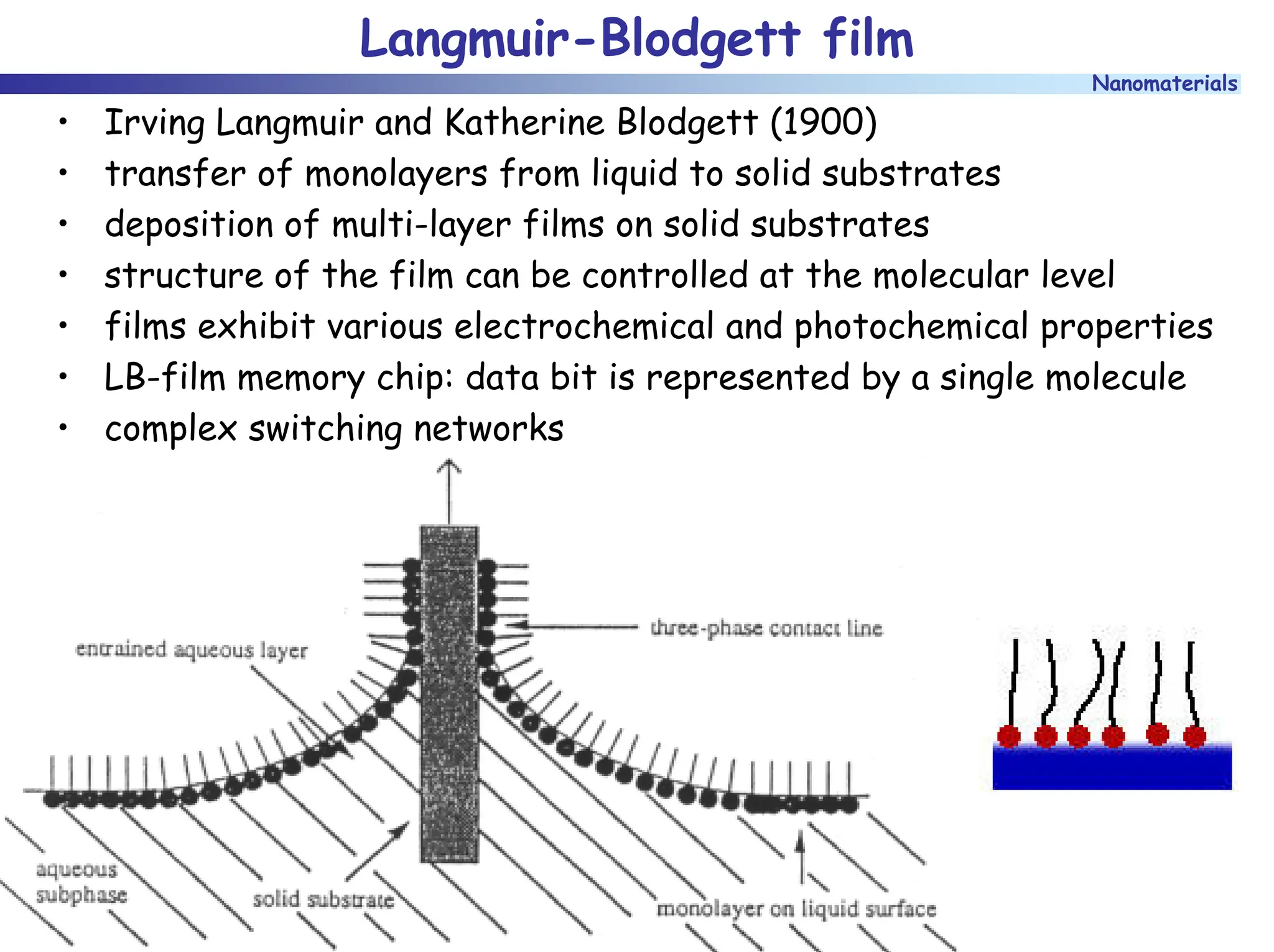
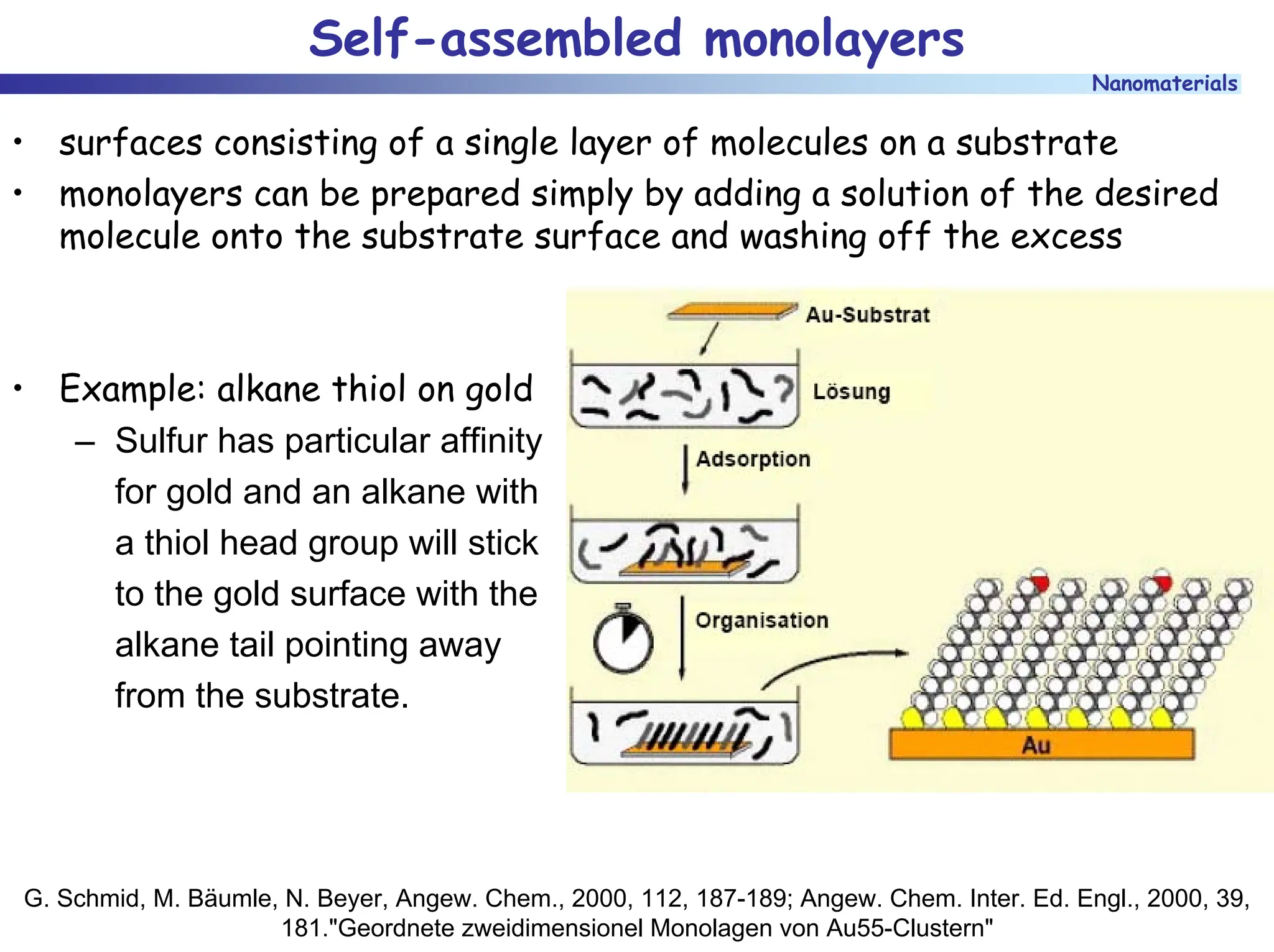
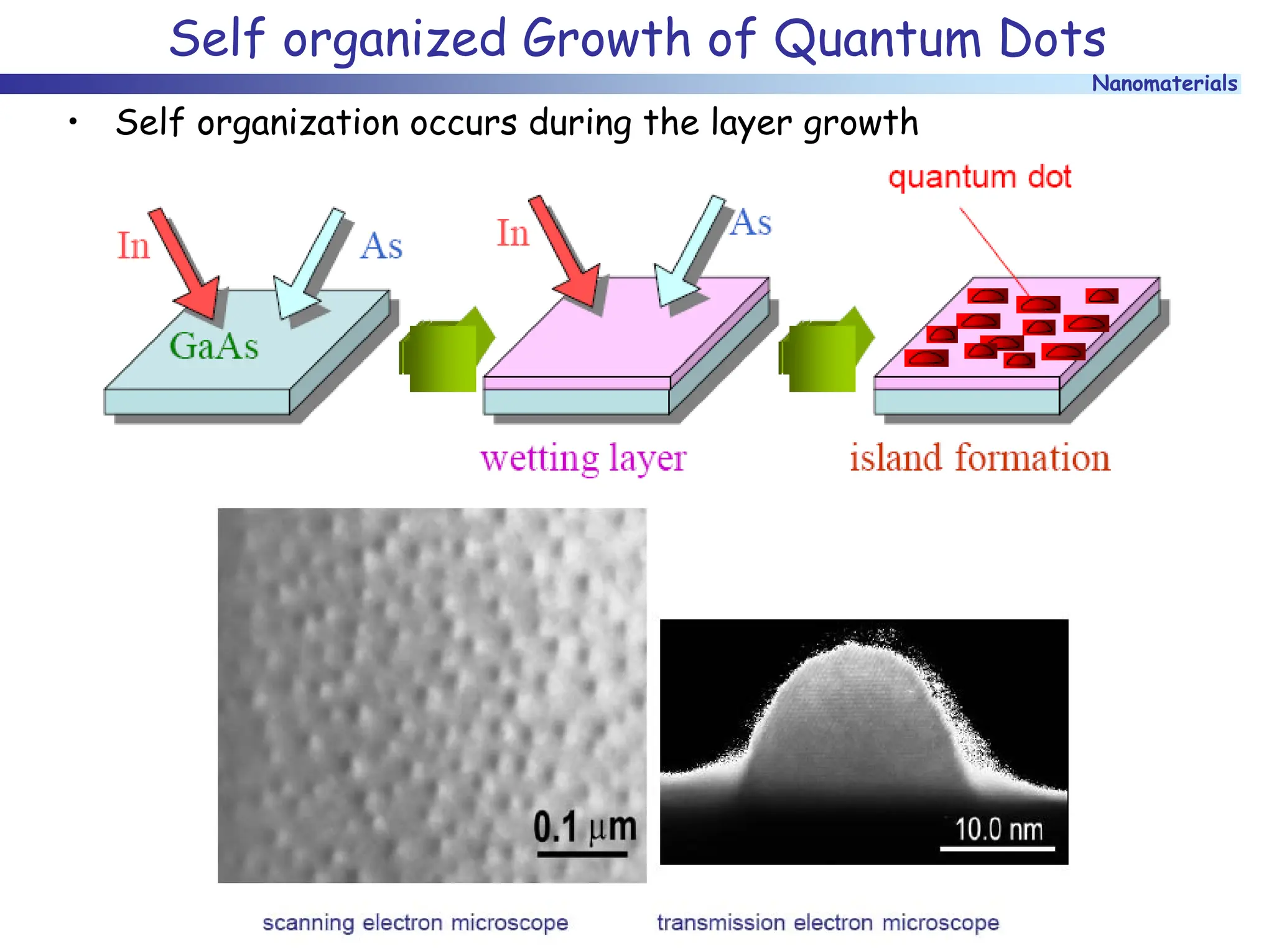
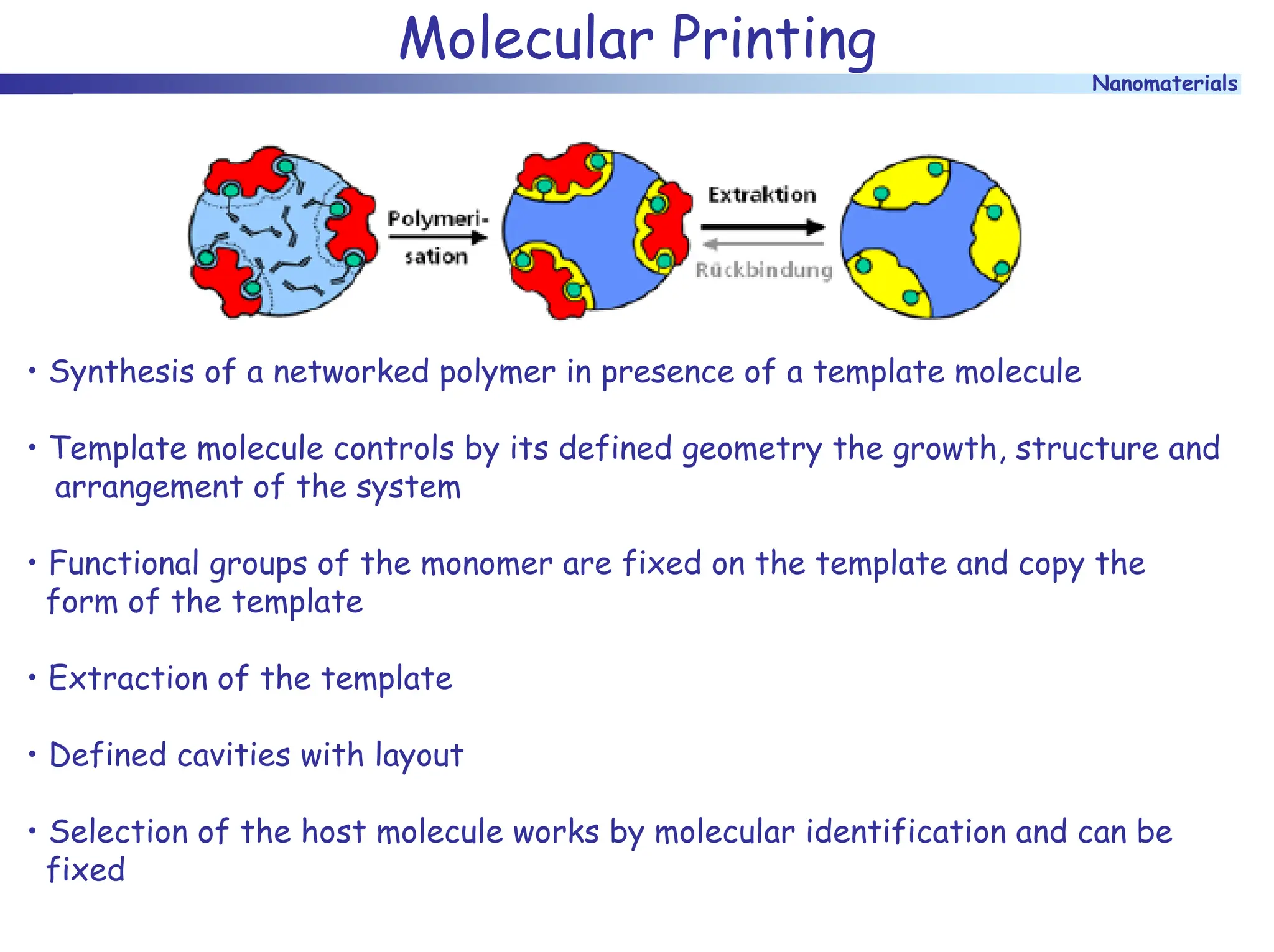
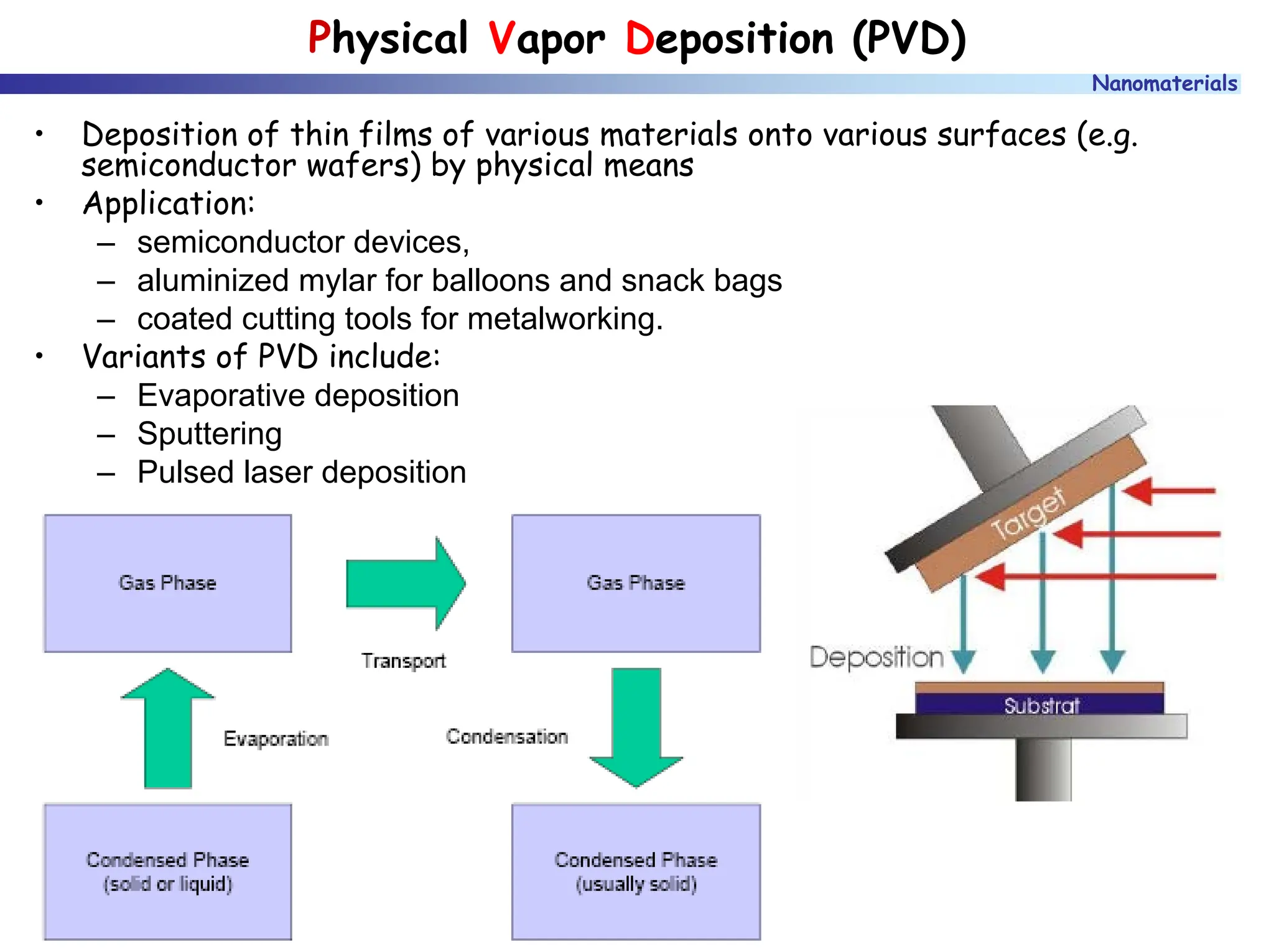
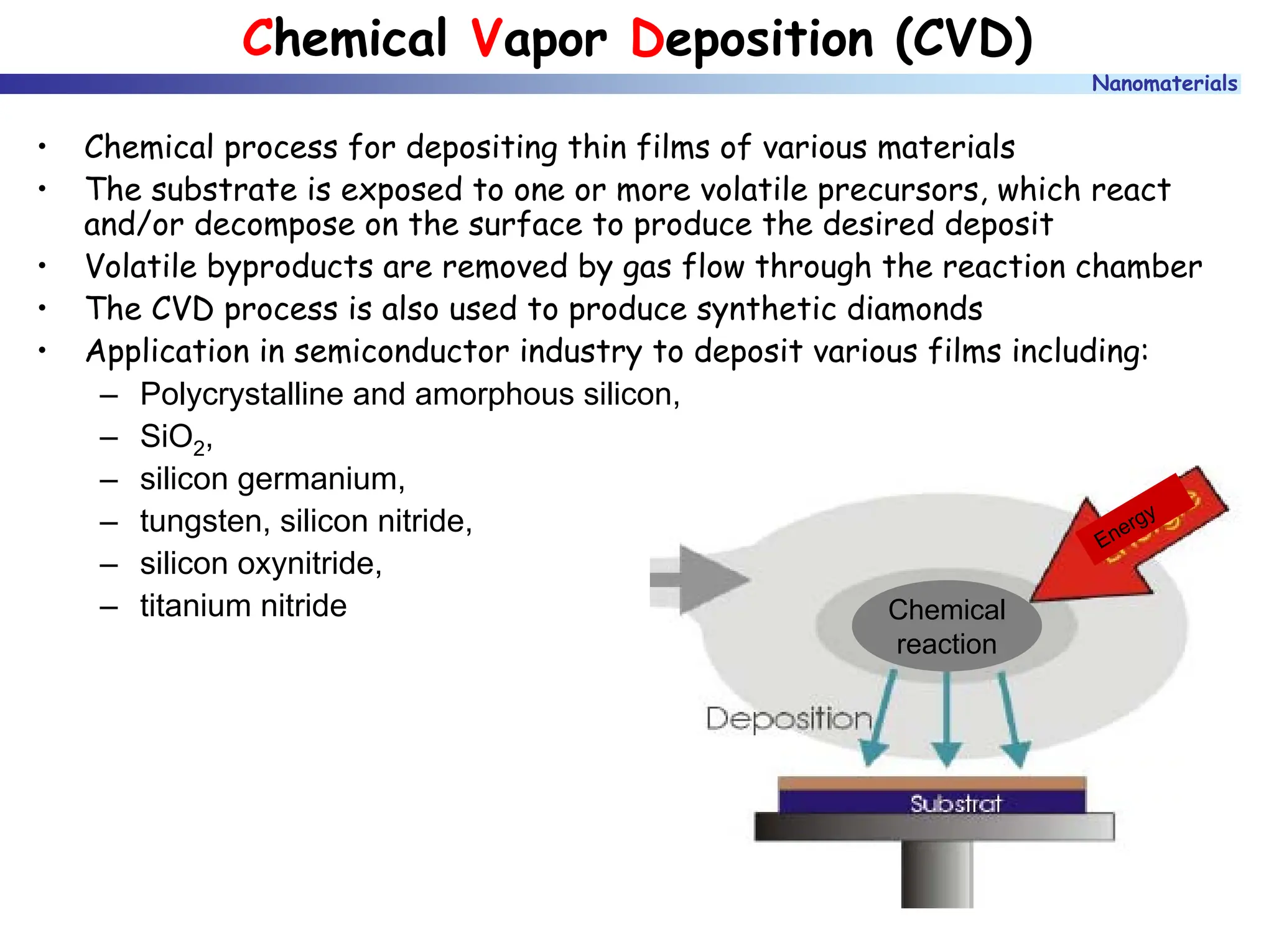
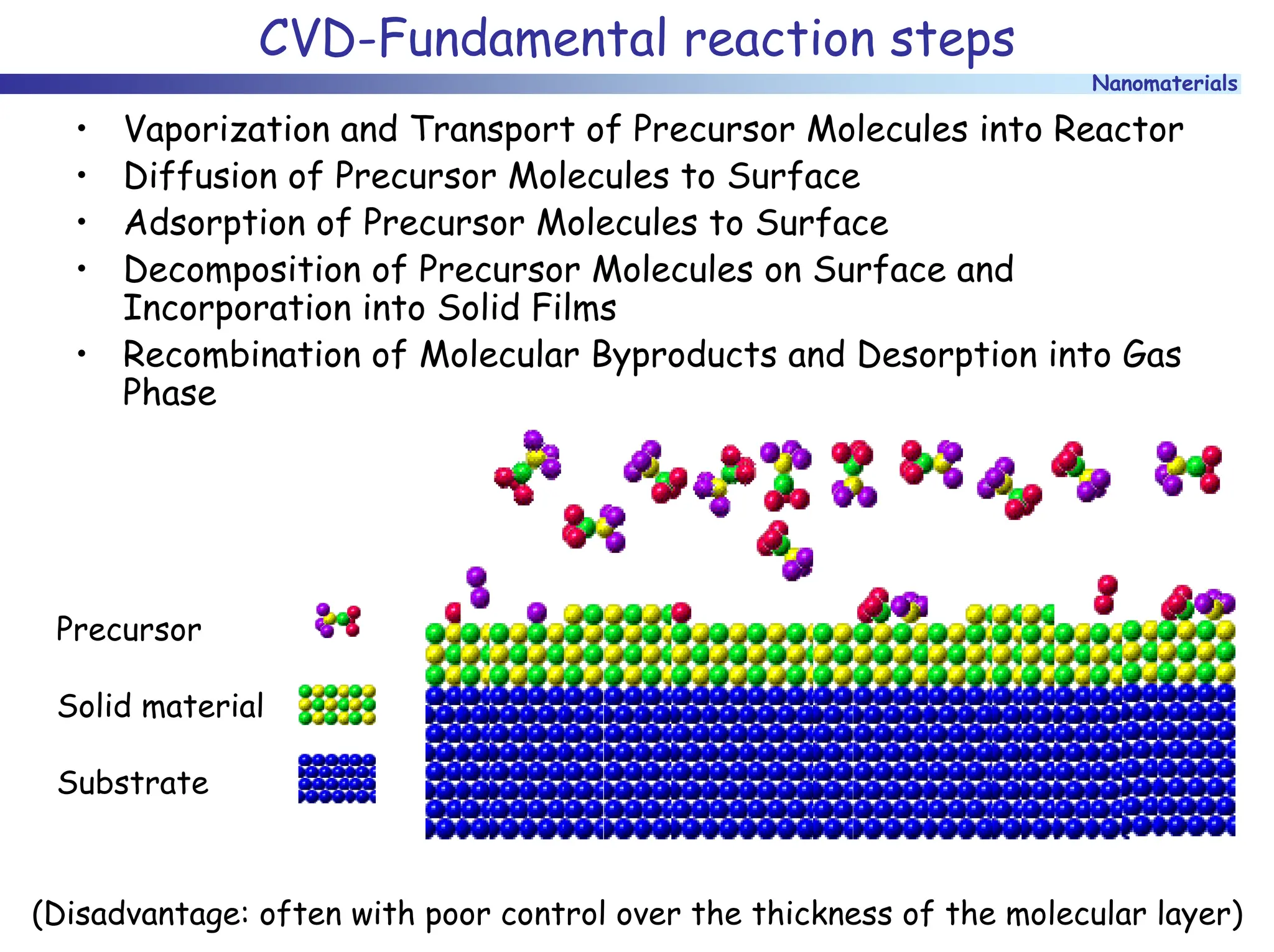
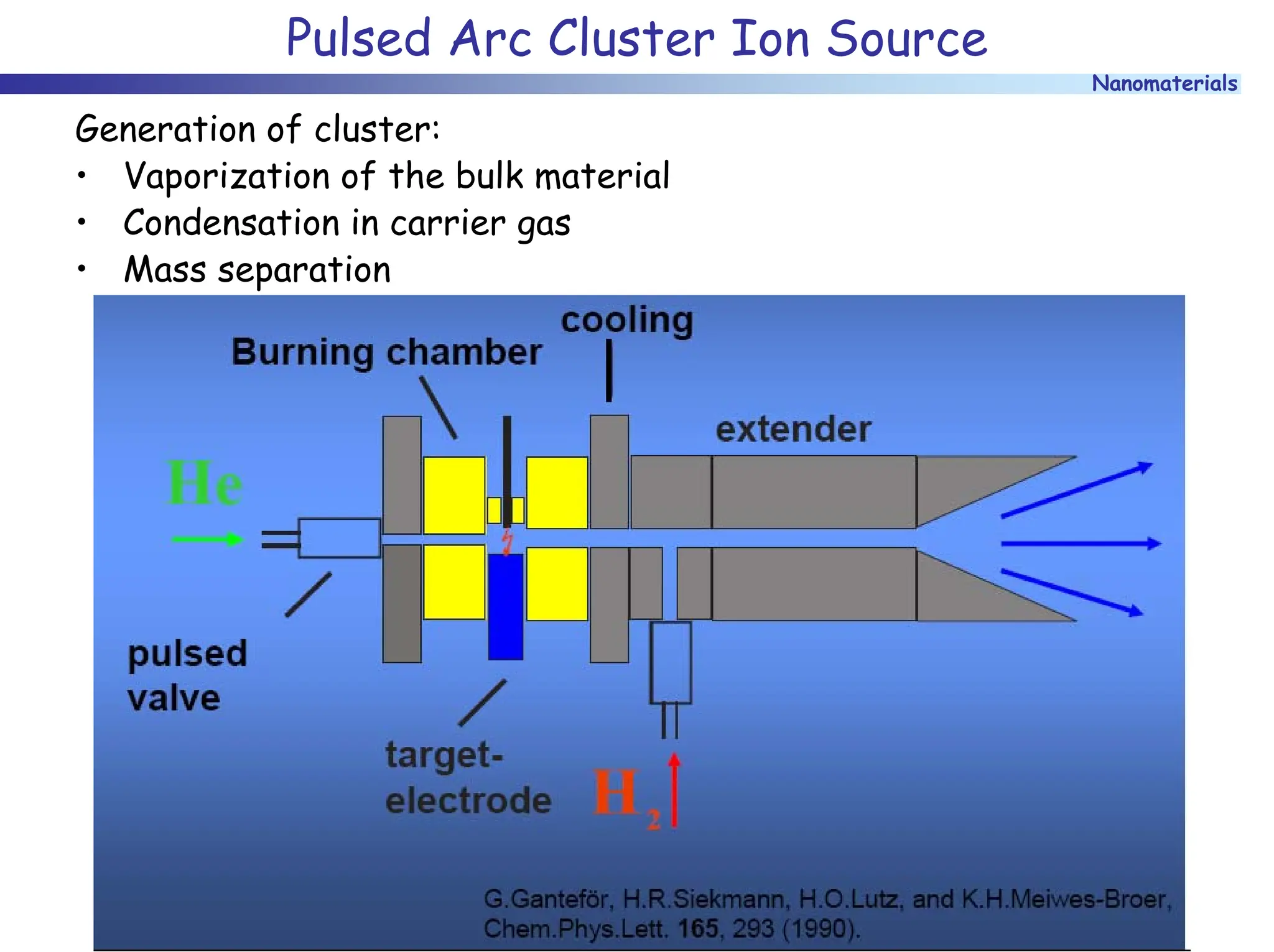
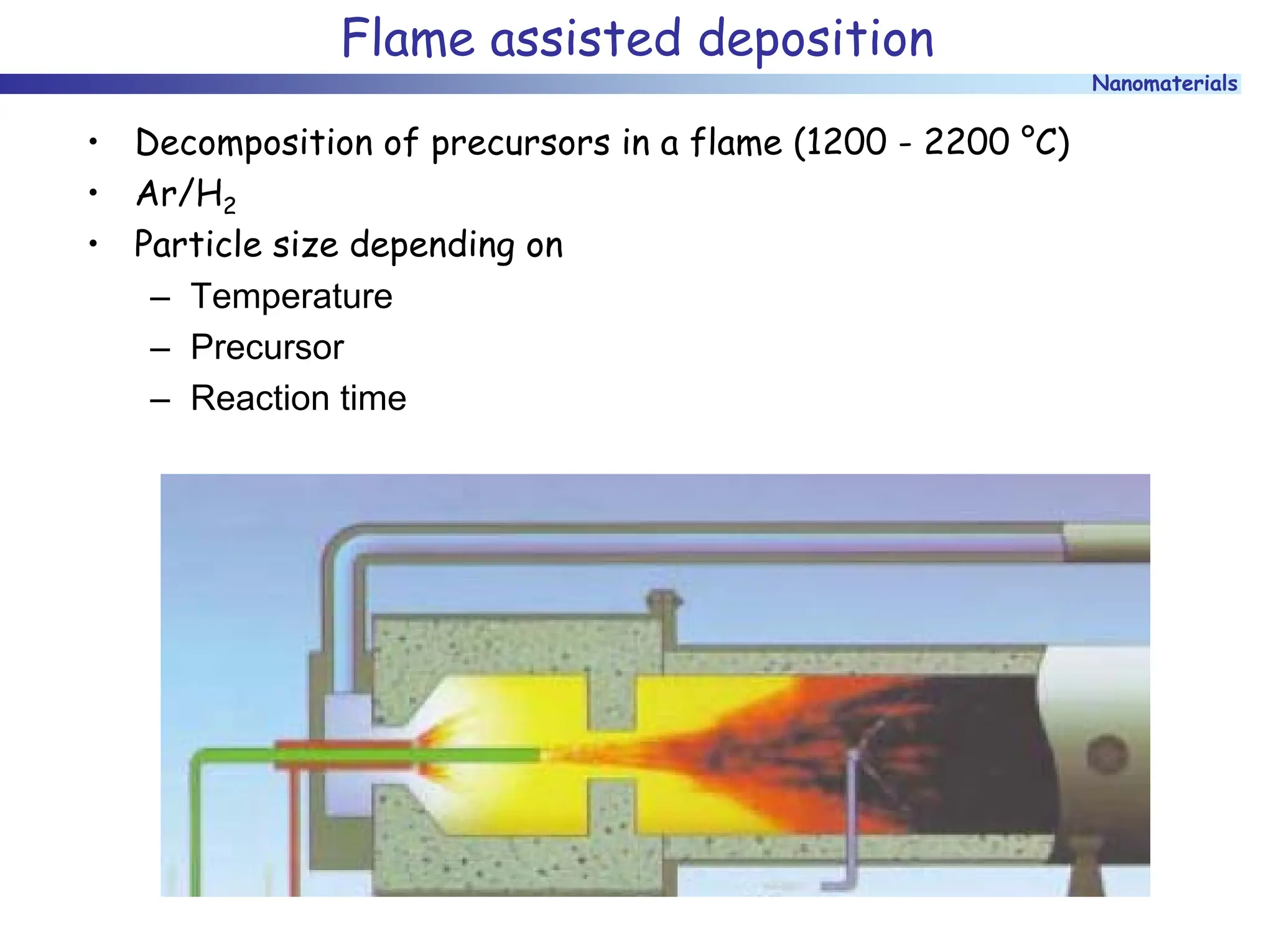
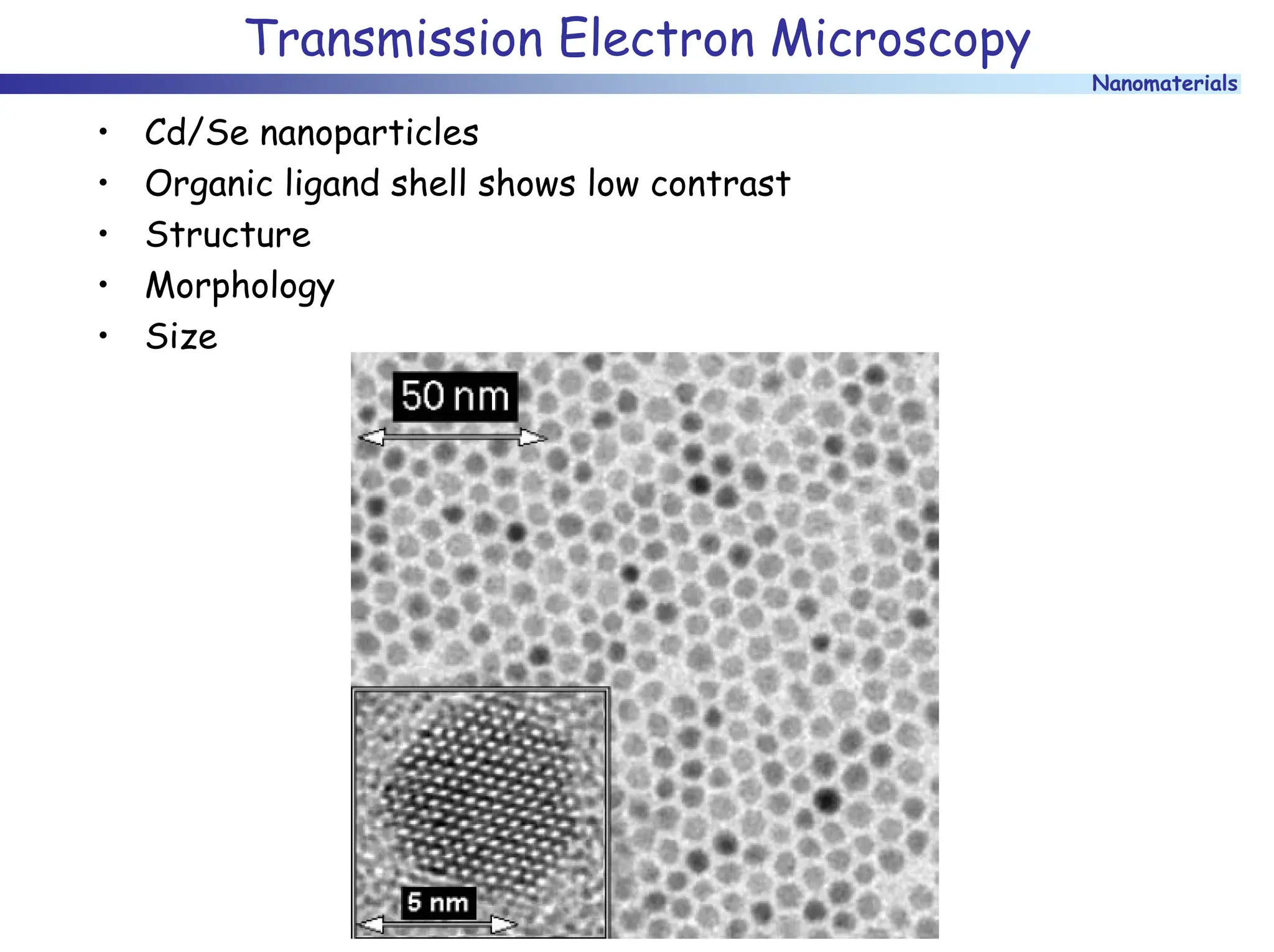
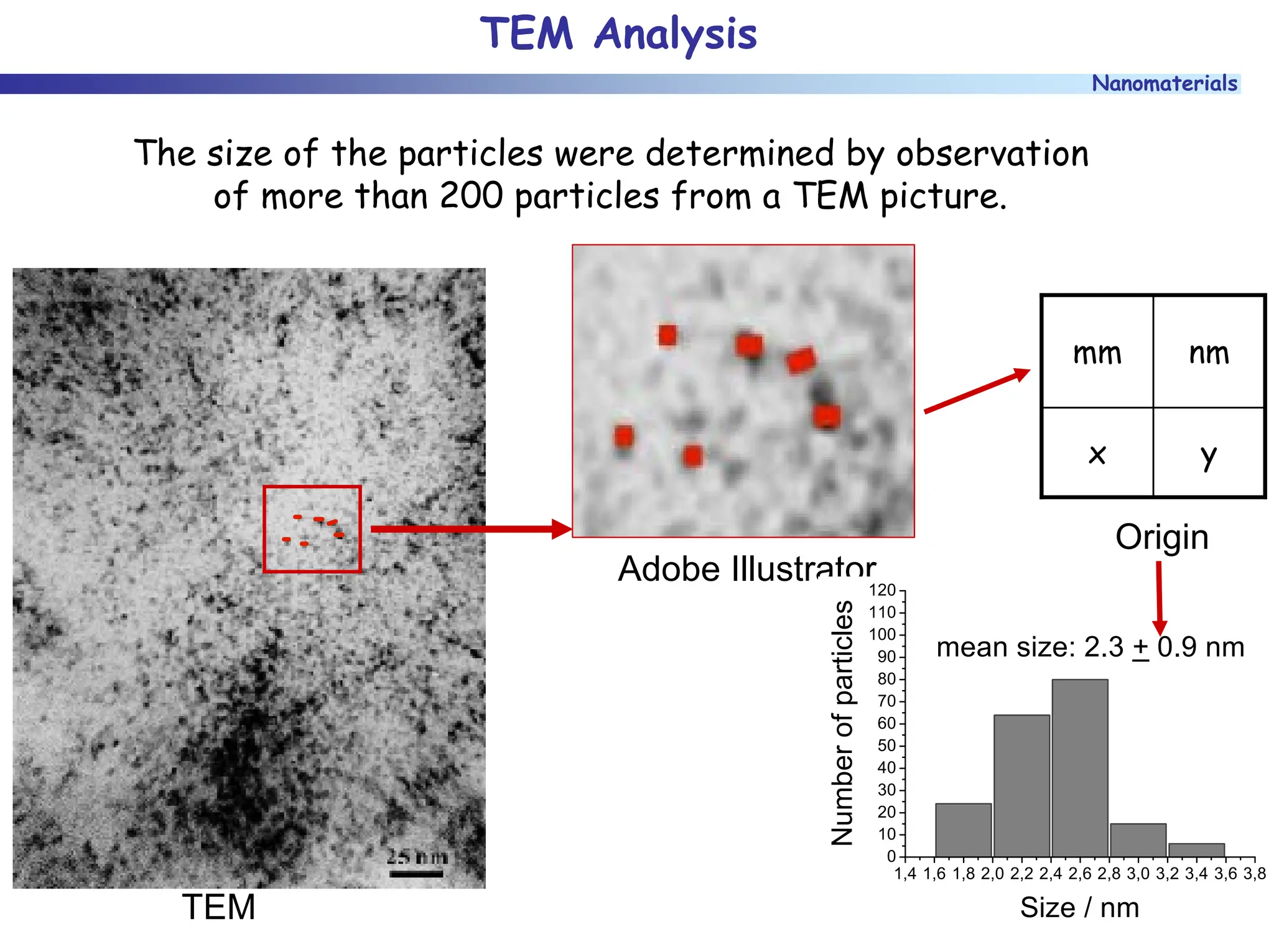
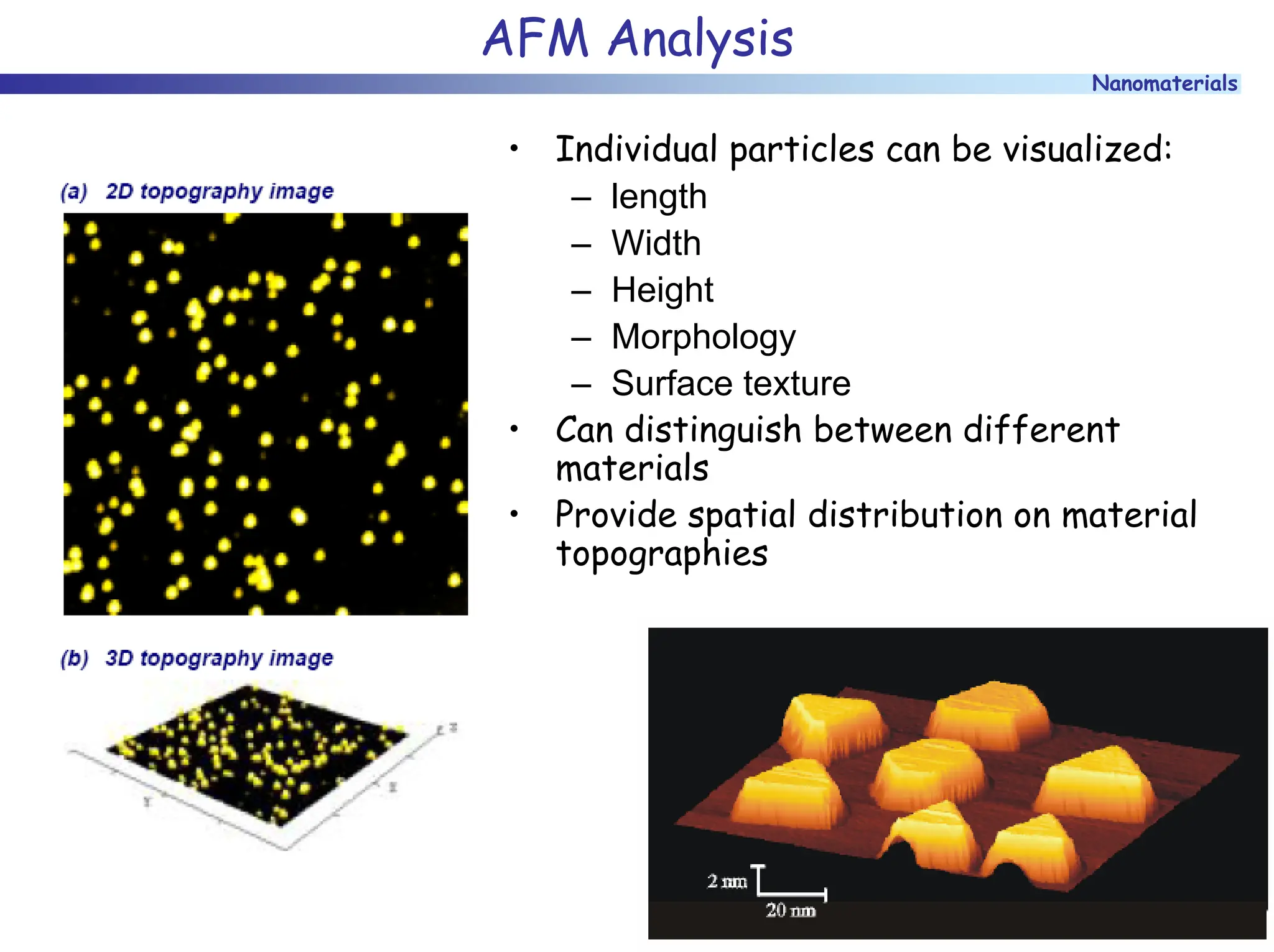
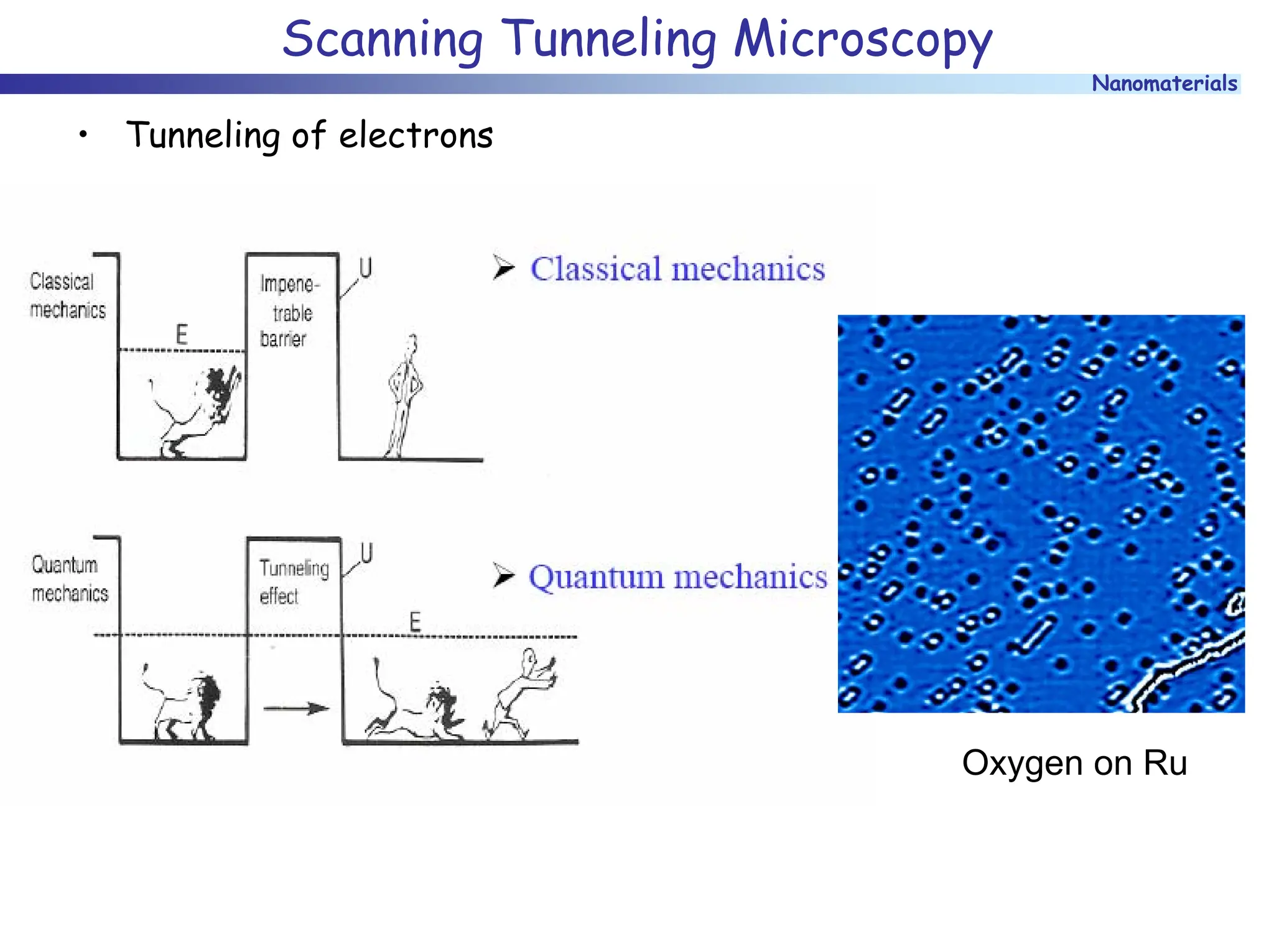
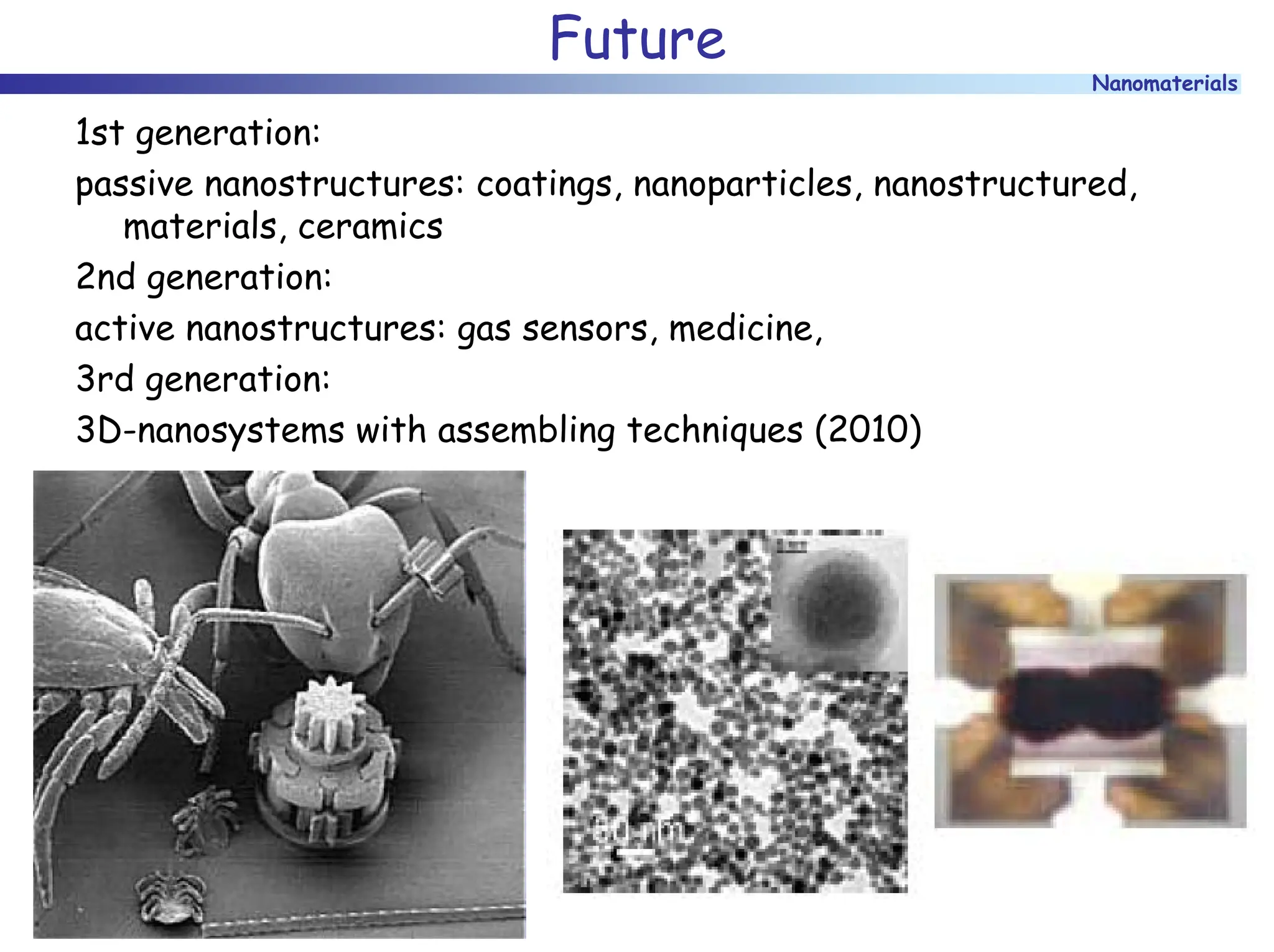
The document discusses nanomaterials, focusing on their physical-chemical properties, classifications, synthesis methods, and diverse applications such as in catalysis and nanoelectronics. It highlights the unique characteristics of nanoparticles, including their size-induced properties, the quantum size effect, and fabrication techniques like sol-gel processes and lithography. Additionally, it describes the importance of stabilization techniques and explores advanced methods for the characterization and analysis of nanostructures.