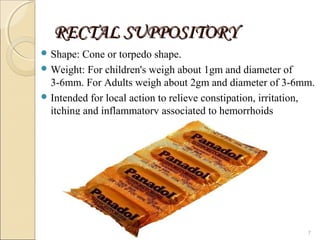
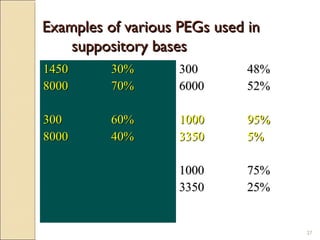
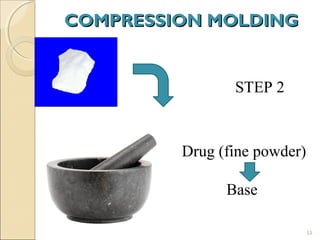
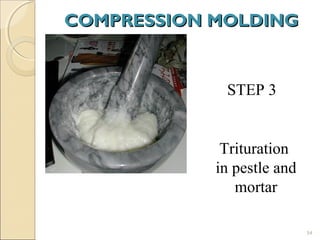
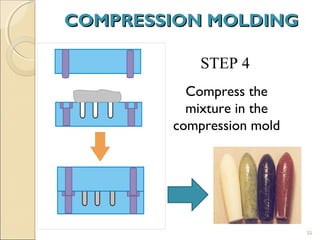
Suppositories are solid dosage forms meant to be inserted into body cavities like the rectum, urethra, and vagina to release drugs locally or systemically. This document classifies suppositories based on their site of insertion and discusses their ideal properties, common bases used, and methods of preparation. The main types are rectal, vaginal, urethral, nasal, and ear suppositories. Common bases include glycerinated gelatin, polyethylene glycols, and cocoa butter, which must meet requirements like melting point and toxicity. Preparation involves mixing the drug with the base and molding or allowing it to solidify.