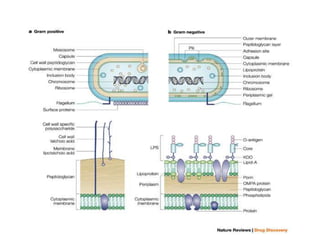
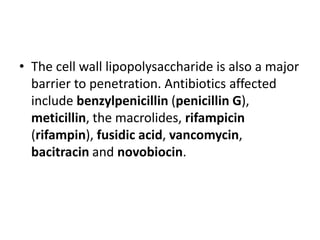
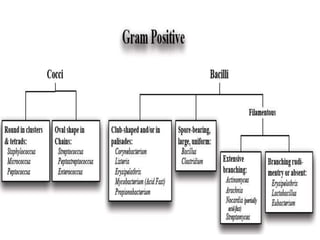
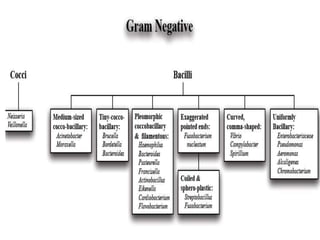
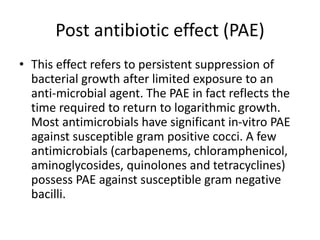
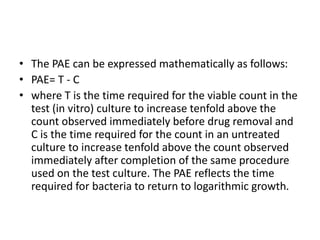
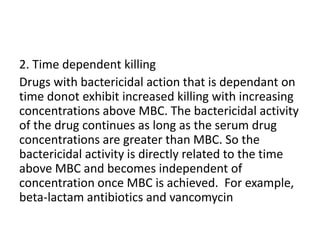
This document discusses chemotherapy and antimicrobial agents. It begins by classifying microorganisms into bacteria, viruses, fungi and parasites. Antimicrobial agents are then classified based on the microorganism they target. Chemotherapy refers to using drugs that are selectively toxic to invading microorganisms. Antibiotics kill or inhibit the growth of microbes. The ideal antimicrobial exhibits selective toxicity against the pathogen without harming the host. Classification of antibiotics is based on their spectrum of activity and biochemical pathway targeted. The document then discusses the cell walls and structures of different microbes and how it impacts antimicrobial penetration. It also covers minimum inhibitory concentrations, post-antibiotic effects, bacterial growth cycles and methods for testing microbial susceptibility.










![MIC
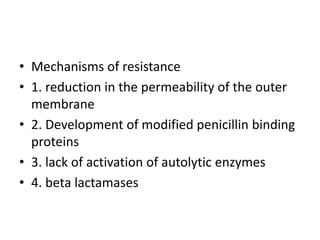
• Testing bacterial pathogens in vitro for their
susceptibility to antimicrobial agents is extremely
valuable in confirming susceptibility, ideally to a
narrow-spectrum nontoxic antimicrobial drug.
Tests measure the concentration of drug required
to inhibit growth of the organism (minimal
inhibitory concentration [MIC]) or to kill the
organism (minimal bactericidal concentration
[MBC]). The lowest concentration of the agent
that prevents visible growth after 18-24 hours of
incubation is known as the minimum inhibitory
concentration (MIC).](https://image.slidesharecdn.com/antibiotics-1-220404071136/85/antibiotics-1-pptx-21-320.jpg)


































































![• Infections with Anaerobes
• Many anaerobic infections are caused by mixtures of
microorganisms. Most are sensitive to penicillin G.
• Pulmonary and periodontal infections (with the
exception of beta-lactamase-producing Prevotella
melaninogenica) usually respond well to penicillin G.
• Mild-to-moderate infections at these sites may be
treated with oral medication (either penicillin G or
penicillin V 400,000 units [250 mg] four times daily).
More severe infections should be treated with 12-20
million units of penicillin G intravenously.](https://image.slidesharecdn.com/antibiotics-1-220404071136/85/antibiotics-1-pptx-108-320.jpg)