Embed presentation
Download to read offline


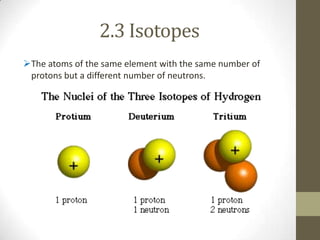
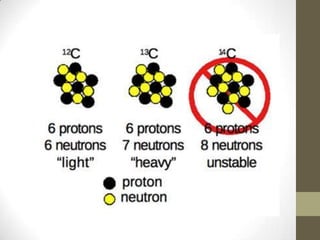
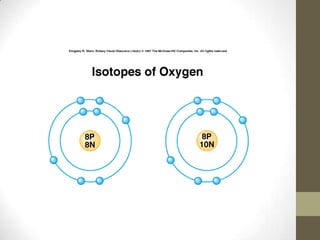
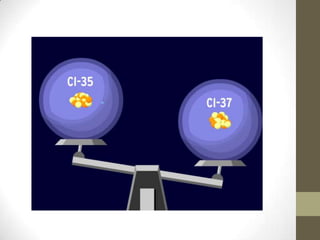
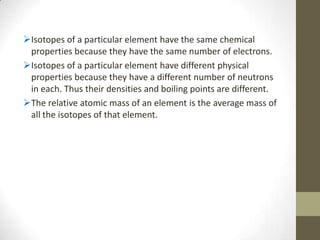

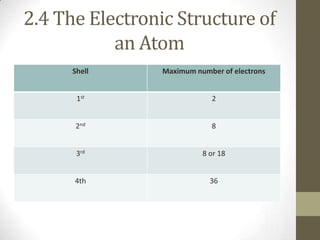

This chapter discusses the structure of the atom, including analyzing matter, synthesizing atomic structure, understanding isotopes and their uses, and appreciating the orderliness and uniqueness of atomic structure. Isotopes of the same element have the same number of protons but different numbers of neutrons. While isotopes have the same chemical properties due to their identical number of electrons, they have different physical properties because of varying neutron counts. The electronic structure of an atom is organized into shells, with the first shell holding up to 2 electrons and higher shells able to accommodate more electrons in set numbers.








