Embed presentation
Download to read offline


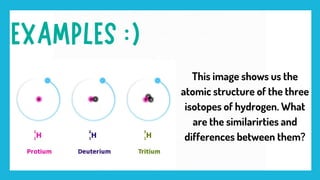




The document provides an overview of isotopes, highlighting their differences from atoms, specifically regarding neutron and proton counts. It explains relative atomic mass as the average mass of an element's isotopes, emphasizing the significance of abundance in this calculation. Additionally, it includes a visual representation of the three isotopes of hydrogen.







