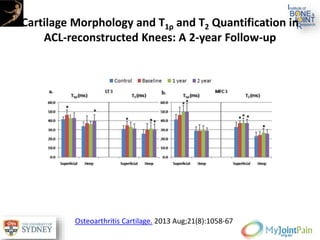
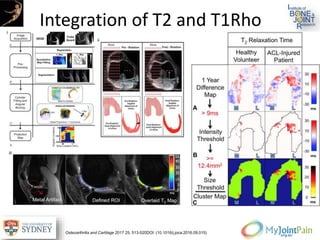
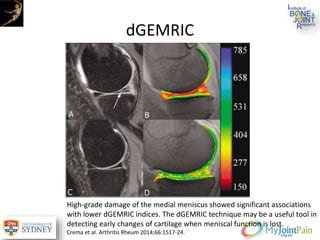
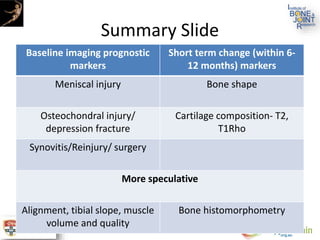
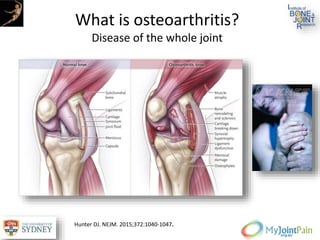
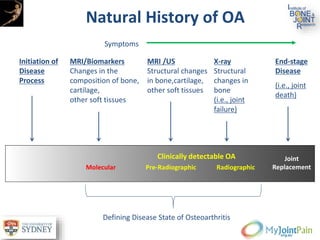
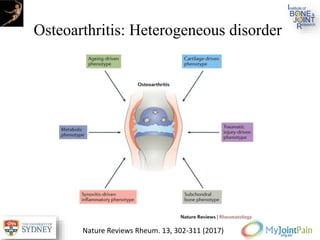
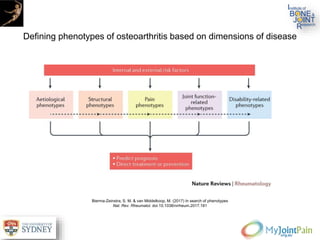
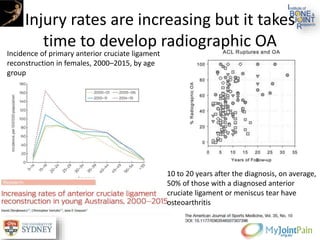
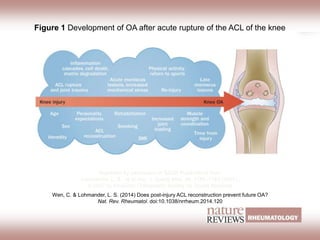
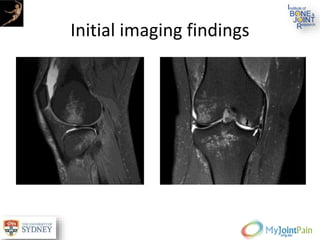
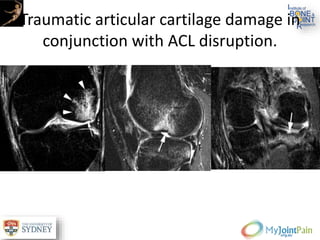
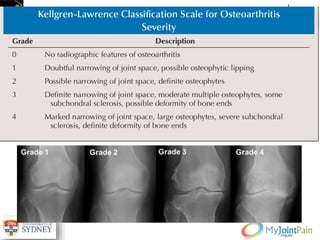
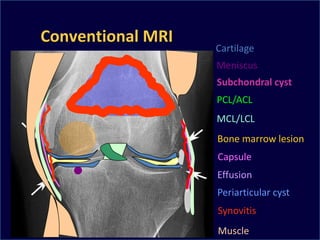
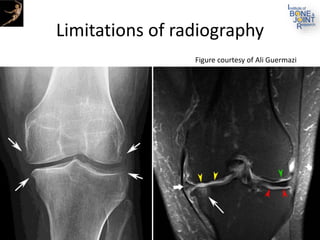
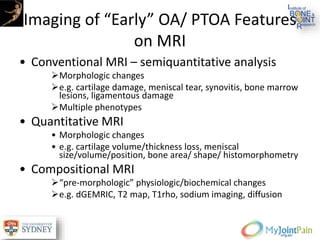
The document presents an extensive review of post-traumatic osteoarthritis (OA) with a focus on structural changes, imaging techniques, and risk factors associated with knee injuries, particularly involving the anterior cruciate ligament (ACL). It highlights the limitations of conventional radiography in detecting early OA and emphasizes the advantages of MRI in identifying pre-radiographic changes. Recommendations include further research on MRI validity and the importance of monitoring meniscal health, synovitis, and cartilage composition for better management and prevention strategies.






![Accepted propositions for definition of
OA on MRI
A definition of tibiofemoral osteoarthritis on MRI would be:
The presence of both group [A] features or one group [A] feature and two or more group [B]
features
Group [A] after exclusion of joint trauma within the last 6 months (by history) and exclusion
of inflammatory arthritis (by radiographs, history and laboratory parameters):
i) Definite osteophyte formation§
ii) Full thickness cartilage loss
Group [B]:
i) Subchondral bone marrow lesion or cyst not associated with meniscal or ligamentous
attachments
ii) Meniscal subluxation, maceration or degenerative (horizontal) tear
iii) Partial thickness cartilage loss (where full thickness loss is not present)
iv) Bone attrition
Definition of PF OA requires all of the following involving the patella and/or anterior femur:
i) A definite osteophyte
ii) Partial or full thickness cartilage loss](https://image.slidesharecdn.com/session002-89014-david-j-190710180358/85/Structural-Targets-for-Prevention-of-Post-Traumatic-OA-21-320.jpg)

![What comes first? Multi-tissue involvement
leading to radiographic osteoarthritis
• Predictors of ROA at P-2:
• Hoffa synovitis (HR 1.76 [95% CI 1.18-2.64])
• Effusion synovitis (HR 1.81 [95% CI 1.18-2.78])
• Medial meniscal damage (HR 1.83 [95% CI 1.17-
2.89]).
• At P -1, all features but meniscal extrusion
predicted radiographic OA, with highest odds for
medial BMLs (HR 6.50 [95% CI 2.27-18.62]) and
effusion synovitis (HR 2.50 [95% CI 1.76-3.54]).
Roemer et al. Arthritis Rheumatol. 2015 May;67(8):2085-96](https://image.slidesharecdn.com/session002-89014-david-j-190710180358/85/Structural-Targets-for-Prevention-of-Post-Traumatic-OA-24-320.jpg)