The document provides information about structural formulas and condensed structural formulas of hydrocarbons:
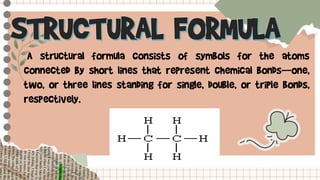
- A structural formula shows the arrangement of atoms in a molecule using lines to represent chemical bonds.
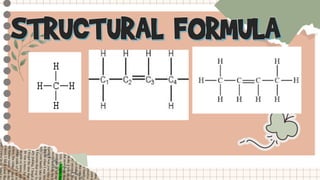
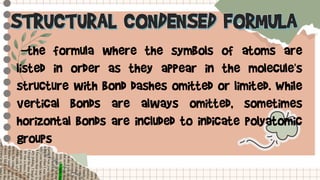
- A condensed structural formula writes the structural formula in a shortened way, often omitting hydrogen atoms and some bonds to reduce clutter.
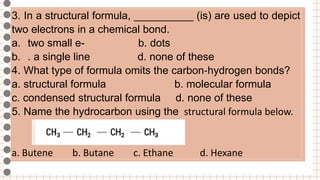
- Examples of tasks involving writing structural formulas, condensed structural formulas, and naming hydrocarbons from their formulas are given.
- The importance of knowing structural and condensed formulas is to understand hydrocarbon molecules and their bonding arrangements.