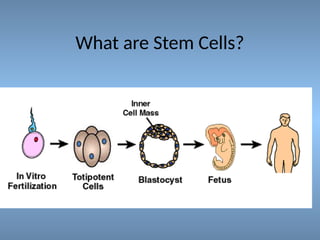
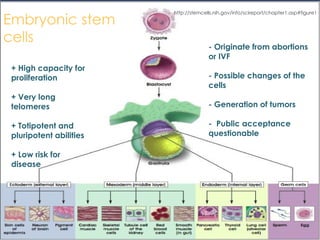
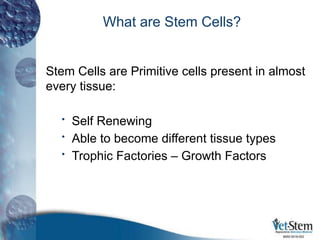
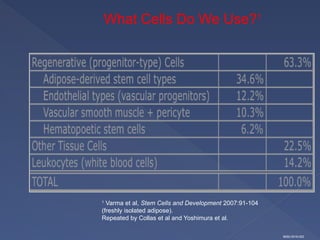
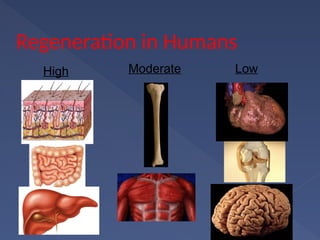
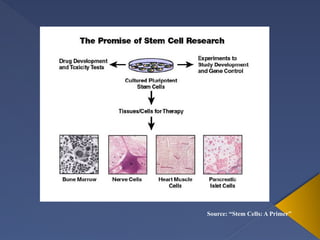
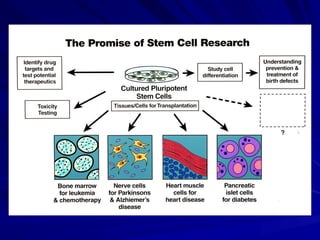
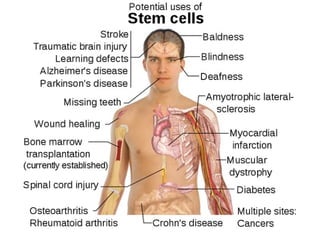
Stem cells are primitive cells that can develop into various tissue types and are crucial for regeneration and healing in the human body. There are two main types: embryonic stem cells, which are pluripotent, and adult stem cells, which are more specialized and harder to isolate. Stem cell research holds potential for treating numerous diseases, understanding growth and development, and developing new pharmaceuticals.