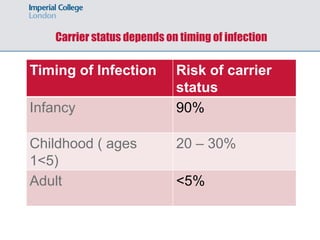
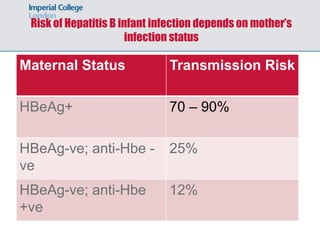
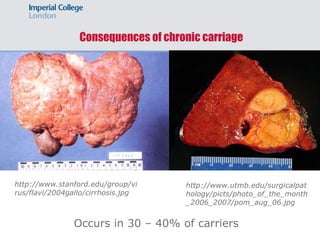
1) Hepatitis B vaccination faces several challenges, including ensuring safety, demonstrating efficacy of recombinant vaccines, determining duration of protection, addressing cost and non-responders.
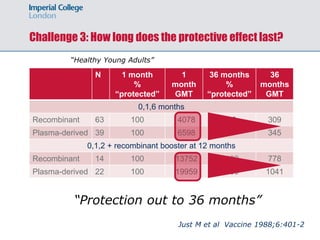
2) Studies showed plasma-derived and recombinant vaccines provided protection for decades, though antibody levels declined over time. Cellular immune responses persisted despite low antibody levels.
3) Global elimination of Hepatitis B is possible by 2090 through high coverage birth dose vaccination, treatment of high-risk groups, and developing a cure for chronic infection. However, this will require significant ongoing financial investment.