spectroscopy-Mass spectroscopy-peinciple,applications
- 1. 1 Mass spectroscopy University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 2. 2 Mass spectrum- a graph of the number of ions detected as a function of their m/z ratio. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 3. Principle 3 To measure relative molecular masses. To know the fragmentation of the molecules. Comparision of mass spectra with known compounds. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 4. Organic molecules are bombarded with electron Converted into highly energetic positively charged ions(Molecular ions or parent ions) Further break up into smaller ions( fragment ions or Daughter ions) The formed ions are separated by Deflection in magnetic field according to their Mass and Charge Mass spectrum 4 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 5. 5 Loss of electron from a molecule leads to radical cation. M e-_ M.+ +e_ radical cation 70eV Fragments Cations radicals neutral molecules radical cations Molecular ion 15eV University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
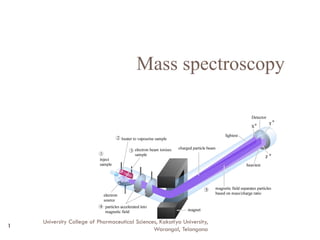
- 6. Instrumentation 6 Inlet system Ion source Ionization methods Mass analyser Ion detector Data system University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 7. Inlet system 7 Solid samples with lower vapour pressure directly inserted into the ionization chamber and volatilization is controlled by heating the probe. Liquids are handled by hypodermic needles Injection through a silicon rubber dam Gases are leaked into the ionization chamber directly by the help of mercury manometer University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 8. Ion source 8 The minimum energy required to ionise an atom or a molecule is called Ionization potential. The common technique used for the production of ion in mass spectrometer is by the bombardment of electrons. The bombarding electrons are produced from an electrically heated tungsten filament. Operating pressure 10-6mm. AB (g)+e AB*+e AB++2e (most propable) AB +n + (n+1).e (least propable) A++B-+e A++B+2e AB- (electron capture-less propable) University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 9. Ionization methods 9 Electron ionization (EI) Chemical Ionization (CI) Field Desorption (FD) Fast Atom bombardment(FAB) Electrospray Ionization (ESI) Matrix Assisted Laser Desorption/Ionization (MALDI) University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 10. 10 Electron ionization (EI) In ES-MS, a beam of high- energy electrons is emitted from a filament that is heated to several thousand degree Celsius. These high- energy electrons strike the stream of molecules that has been admitted from the sample inlet system. The electron- molecule collision strips an electron from the molecule, creating a cation. A repeller plate, which carries a positive electrical potential, directs the newly created ions toward a series of accelerating plates. A large potential difference, ranging from 1 to 10 kilovolts (kV), applied across these accelerating plates produces a beam of rapidly traveling positive ions. One or more focusing slits direct the ions into a uniform beam. The energy required to remove an electron from an atom or molecule is its ionization potential or ionization energy. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 11. 11 Electron ionization (EI) University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 12. 12 Chemical Ionization(CI) In CI-MS, the sample molecules are combined with a stream of ionized reagent gas that is present in great excess relative to the sample. When the sample molecules collide with the pre ionized reagent gas, some of the sample molecules are ionized by various mechanisms, including proton transfer, and adduct formation. Common ionizing reagents for CI-MS include methane, ammonia, isobutane and methanol. The vaporized sample is introduced into the mass spectrometer with an excess of a reagent gas ( methane) at pressure of about 1 torr. The excess carrier gas is ionized by electron impact to the primary ions These may react with the excess methane to give secondary ions. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 13. CH4 .+ + CH4 CH5 + and CH3. CH3 + + CH4 C2H5 + and H2 CH4 + C2H5 + C3H5 + + H2 CH3 + + M [M + H]+ + CH4 C2H5 + + M [M + H]+ + C2H4 13 These react with excess methane to give secondary ions. The secondary ions react with the sample(M) University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 14. 14 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 15. Field Desorption(FD) 15 Stable molecular ions are obtained from a sample of low volatility, which is placed on the anode of a pair of electrodes, between which there is an intense electric field. Desorption occurs, and molecular and quasimolecular ions are produced with insufficient internal energy for extensive fragmentation. Usually master peak is represented by [M+H]+ ion. Synthetic polymers with molecular weights on the order of 10,000Da have been analyzed, but there is a much lower molecular weight limit for polar biopolymers; here FAB procedure and others are more superior. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 16. 16 Field Desorption(FD) University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 17. Fast Atom Bombardment (FAB) 17 Polar molecules such as peptides, with molecular weights up to 10,000 Da can be analyzed by a “soft” ionization techniques called fast atom bombardment (FAB).The bombarding beam consists of xenon (or argon) atoms of high translational energy (Xe). This beam produced by first ionizing xenon atoms with electrons to give xenon radical cations: Xe Xe.+ +2e The radical cations are accelerated to 6-10 keV to give radical cations of high translational energy (Xe).+which are then passed through xenon. During this passage, the charged high energy xenon obtains electrons from the xenon atoms to become high energy atoms(Xe), and the Xe.+ ions are removed by an electric field. Xe.+ Xe.+ Xe.+ +Xe Xe+ Xe.+ The compound of interest is dissolved in a high boiling viscous solvent such as glycerol; a drop is placed on a thin metal sheet, and the compound is ionized by the high energy xenon atoms(Xe).Ionization by translational energy minimizes the amount of vibrational excitation, and this results in less destruction of the ionized molecules.The polar solvent promotes ionization and allows diffusion of fresh sample to the surface. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 18. 18 Fast Atom Bombardment (FAB) University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 19. Electrospray Ionization (ESI) 19 ESI involves placing an ionizing voltage-several kilovolts- across the nebulizer needle attached to the outlet from a high performance liquid chromatograph (HPLC). This technique is used for water- soluble bio molecules- proteins, peptides and carbohydrates in particular. The result is a spectrum whose major peaks consist of the molecular ion with a different number of charges attached. A molecular ion of, for example, about 10,000 Da with a charge (z) of 10 would behave in a mass spectrometer as though its mass were about 1000 daltons. Its mass, therefore, can be determined with a spectrometer of modest resolution- and cost. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 20. 20 Electrospray Ionization (ESI) University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 21. 21 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 22. 22 Professor John B. Fenn University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 23. Atomic pressure ionization(API) 23 It is a simple variation of ESI. It is applied to the outlet of an HPLC unit attached to the inlet of the mass spectrometer. These variations have in common the formation of a very fine spray (nebulization) from which the solvent can be quickly removed. The small particles are then ionized by a corona discharge at atmospheric pressure and swept by the continuous flow of the particles and a small electric potential that moves the positively charged particles through a small orifice into the evacuated mass spectrometer. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 24. 24 Atomic pressure ionization(API) University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 25. Matrix Assisted Laser desorption/Ioniztion (MALDI) 25 It is mainly used for large bio molecules. The sample in a matrix is dispersed on a surface, and is desorbed and ionized by the energy of a laser beam. The matrix serves the same purpose as it does in the FAB procedure. This procedure is recently used in several variations to determine the molecular weight of large protein molecules- up to several hundred kDa. The combination of a pulsed laser beam and a time –of flight mass spectrometer. Used in peptide sequencing. Matrix selection is critical and depends on the wavelength of the laser beam and on the nature of the sample. Such as polar compounds as carboxylic acids (e.g. nicotinic acid), urea and glycerol. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 26. 26 Matrix Assisted Laser desorption/Ionization (MALDI) University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 27. 27 Ionizing agents in Mass spectrum Name and Acronym Ionizing agents Compounds Mass range Electron Impact (EI) Energetic electrons Thermally volatile and stable 500Da Chemical Ionization (CI) Reagent gaseous Thermally volatile and stable 500Da Fast atom bombardment (FAB) Energetic atomic beam Peptides 7000Da Field Ionization (FI) High –potential electrode Thermally volatile 1000Da Field Desorption (FD) High –potential electrode Biopolymers 10000Da Electospray ionization (ESI) High electrode field Polar and basic 7000Da Matrix –assisted Laser desorption ionization(MALDI) Laser beam Laser bio molecules 3,00,000Da Atomic Pressure Ionization (API) Energetic atomic beam Thermally liable 1000Da University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 28. 28 Mass analyser Dempster’s kinetic energy 1/2.mV2=eV --------------(1) Where , v=velocity of the ions after acceleration V=potential applied From Newton’s second law of motion H eV=mv2/r --------------(2) Squaring both sides H2e2v2=m2v4/r2 H2e2=m2v2/r2 --------------(3) But ½ mv2= eV mV2=2eV Putting the value of mv2 in (3) H2e2=m,.2eV/r2 or H2e= 2mV/r2 Or m/e= H2r2/2V University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 29. 29 The mass spectrum can be obtained either by (i) Changing H at constant V or (ii) Changing V at constant H University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 30. mass analyzers 30 Double –focusing mass analyzer Quadrapole mass analyzer Quadrapole Ion storage (Ion trap) Time-of- flight mass analyzer University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 31. Double –focusing mass analyzer 31 The introduction of an electrostatic field after the magnetic field permits high resolution so the mass of a particle can be obtained to four decimal places. Ions generated in the source are accelerated toward the analyzer. The magnetic field provides directional focusing. The path of the positive ion is again curved by the electric field applied perpendicular to the flight path of the ions. This double focusing provides resolution as high as 60,000. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 32. 32 Double –focusing mass analyzer University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 33. Quadrapole mass analyzer 33 This mass filter uses four voltage- carrying rods. Ions entering from one end travel with constant velocity in the direction parallel to the poles (z direction), but acquire complex oscillations in the x and y directions by application of both a direct current (dc) voltage (Vdc)and a radiofrequency (rf) voltage (V rf) to the poles. There is a “stable oscillation” that allows a particular ion to pass from one end of the quadrapole to the other without striking the poles; this oscillation is dependent on the m/z ratio of an ion. Therefore, ions of only a single m/z value will traverse the entire length of the filter at a given set of conditions. All other ions will have unstable oscillations and will strike the poles and be lost. Mass scanning is carried out by varying each of rf and dc frequencies keeping their ratios constant. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 34. 34 Quadrapole mass analyzer University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 35. 35 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 36. + + + - - 36 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 37. + + + - - 37 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 38. + + + - - Splat 38 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 39. + - - + + 39 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 40. + - - + + 40 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 41. Quadrapole Ion storage (Ion trap) 41 The ion storage trap is a spherical configuration of the linear quadrapole mass filter. The operations are differ in that the linear filter passes the sorted ions directly through to the director, whereas the ion trap retains the unsorted ions temporarily within the trap. They are then released to the director sequentially by scanning the electric field. These instruments are compact (bench top), relatively inexpensive, convenient to use, and very sensitive. They also provide an inexpensive method to carry out GC/MS/MS experiments. In general, the quadrapole instruments do not achieve the mass range and the high resolution of the sector instruments. However, the mass range and resolution are adequate for unit-resolution mass spectrometry, and the rapid scan and sensitivity make them especially suitable for use with capillary gas chromatography. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 42. 42 Quadrapole Ion storage (Ion trap) University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 43. Time-of- flight mass analyzer 43 In the time-of-flight (TOF) mass spectrometers, all singly charged particles subjected to a potential difference V attain the same translational energy in electron volts (eV). Thus lighter particles have the shorter TOF over a given distance. The accelerated particles are passed into a field –free region where they are separated in time by their m/z values and collected. Since arrival times between successive ions can be less than 10-7s, fast electronics are necessary for adequate resolution. Time-of –flight devices are used with sophisticated ionizing methods(FAB, laser desorption, and plasma desorption) University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 44. 44 Time-of- flight mass analyzer University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 45. Detectors 45 Faraday cup Electron Multiplier Photomultiplier University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 46. 46 Faraday cup detector The basic principle is that the incident ion strikes the dynode surface which emits electrons and induces a current which is amplified and recorded. The dynode electrode is made of a secondary emitting material like CsSb,GaP or BeO. It is ideally suited to isotope analysis University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 47. Electron multipliers 47 Electron multipliers are the most common especially when positive and negative ions need to be detected on the same instrument. Dynodes made up of copper-beryllium which transduces the initial ion current and electron emitted by first dynode are focused magnetically from dynode to the next. Final cascade current is amplified more than million times. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 48. 48 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 49. Photomultiliers 49 The dynode consists of a substance ( a scintillator) which emits photons (light). The emitted light is detected by photo multiplier tube and is converted into electric current. These detectors are useful in studies on metastable ions. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 50. Types of peaks 50 Molecular ion peak Fragment ion peak Rearrangement ion peak Metastable ion peak Multicharged ion peak Base peak Negative ion peak University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 51. 51 Molecular ion peak: When a sample is bombarded with electrons of 9 to 15eV energy, the molecular ion is produced, by loss of single electron. M M+ + 2e_ Fragment ions peak: When an energy is given further more upto 70 eV, fragment ions produced, it have lower mass number. Rearrangement ion peak: Recombination of fragment ion is known as rearrangement peaks Metastable ion peaks: The ions resulting from the decomposition between the source region and magnetic analyser are called as Metastable ions. These appear as broad called metastable ion peaks. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 52. 52 Multicharged ions: Ions may exist with 2 or 3 charges instead of usual single charge. The peaks due to these charged ions are known as Multicharged ion peaks. Base peak: The largest peak in the mass spectrum corresponding to the most abundant ion or most intense peak in the spectrum is called as base peak. Negative ion peak: Negative ions are formed from electron bombardment of sample. These results due to the capture of electron by a molecule during collision of molecules. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 53. Mass interpretation 53 Fragmentation rules Mc Lafferty rearrangement Alpha cleavage Beta cleavage Nitrogen rule Retro diel’s alder reaction IHD University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 54. 54 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 55. Fragmentation rules 55 Rule 1: The relative height of the molecular ion peak is greatest for the straight –chain compound and decreases as the degree of branching increases. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 56. 56 Rule2: The relative height of the molecular ion peak usually decreases with increasing molecular weight in a homologous series. Fatty esters appear to be an exception. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 57. 57 Rule 3: Cleavage is favored at alkyl- substituted carbon atoms; the more likely is cleavage. This is a concequence of the increased stability of a tertiary carbocation over a secondary which in turn is more stable than a primary. Cation stability order: Generally, the largest substituent at a branch is eliminated most readily as a radical, presumably because a long-chain radical can achieve some stability by delocalization of the lone electron. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 58. 58 Rule 4: Double bonds, cyclic structures, and especially aromatic (or hetero aromatic) rings stabilize the molecular ion and thus increase the probability of its appearance. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 59. 59 Rule 5: Double bonds favors allylic cleavage and give the resonance-stabilized allylic carbocation. This rule does not hold for simple alkenes because of the ready migration of the double bond, but it does hold for cycloalkenes. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 60. 60 Rule 6: Saturated rings tend to lose alkyl side chains at the α bond. This is merely a special case of branching (rule 3). The positive charge tends to stay with the ring fragment. Unsaturated rings can undergo a retro-Diels –Alder reaction University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 61. 61 Rule 7: In alkyl- substituted aromatic compounds, cleavage is very probable at the bond β to the ring, giving the resonance- stabilized benzyl ion or, more likely, the tropolium ion. Eg. Mass spectra of n-butyl benzene University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 62. 62 Rule 8: The C-C bonds next to a heteroatom are frequently cleaved, leaving the charge on the fragment containing the heteroatom whose nonbonding electrons provide resonance stabilization. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 63. 63 Rule 10: Cleavage is often associated with elimination of small, stable, neutral molecules, such as carbon monoxide, olefins, water, ammonia, hydrogen sulfide, hydrogen cyanide, mercaptans, ketenes, or alcohols, often with rearrangement. Eg. McLafferty rearrangement University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 64. McLafferty rearrangement 64 McLafferty arrangement can occur in ketones, aldehydes, carboxylic acids and esters. In this rearrangement a radical center in molecular ion derived from a lone pair or pi bond, removes hydrogen from the Gamma position (y), a pi bond is formed between the β and y position, and the bond between the α and β positions is broken. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 65. 65 C H3 O R C H2 OH +. + C H2 CH2 C H3 CH2 C H3 CH2 +. + C H2 CH2 CH3 O CH2 + O H CH2 CH3 ]+. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 66. 66 Double Mc Latterfty Rearrangement C H3 O R C H2 OH +. + C H2 CH2 C H3 CH2 C H3 CH2 +. + C H2 CH2 CH3 O CH2 + O H CH2 CH3 ]+. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 67. Αlpha cleavage: Radical-site-initiated cleavage 67 Alpha cleavage in mass spectrometry is a characteristic fragmentation of the molecular ion derived from carbonyl compounds, in which the bond linking the carbonyl carbon to the atom occupying an alpha position breaks. It is an expected pathway for carbonyl compounds, ethers, halides, alcohols and amines. Write the reaction University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 68. Charge- site- initiated cleavage: Inductive cleavage 68 Inductive cleavage involves the attraction of an electron pair by an electronegative heteroatom that ends up as a radical or as a closed-shell neutral molecule. While α-cleavage is a fragmentation of OE+ only, inductive cleavage can operate on OE+ or an EE+. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 69. Βeta cleavage 69 Beta cleavage in mass spectrometry is a characteristic fragmentation of the molecular ion derived from some organic compounds, most notably alcohols, ethers and amines, in which the bond connecting alpha- and beta- carbons break. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 70. Retro-diels- Alder reactions 70 Unsaturated six-membered rings can undergo a retro Diels- Alder fragmentation to produce the radical cation of a diene and a neutral alkene- the hypothetical precursors to the cyclohexene derivative if it had been prepared in the forward direction via the [4pi+2pi] diene + dienophile cycloaddition known as Diels Alder reaction. C H2 CH2 + C H2 CH2 -e + + University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 71. Two- bond cleavage 71 In this process, an elimination occurs, and the odd – electron molecular ion yields an OE+ and an even - electron neutral fragment N, usually a stable small molecule of some type :H2O, a hydrogen halide, or an alkene. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 72. Alkanes 72 The relative height of the parent peak decreases as the molecular mass increases in the homologous series. Groups of peaks in the mass spectrum are observed 14 mass units apart. The most abundant peaks correspond to CnH2n+1 ion. The most intense peaks are due to C3 and C4 ions at m/e 43 and m/e 57 respectively. There is no preferred charge stabilization site to favor any specific cleavage. The peaks corresponding to CnH2n+1 ions are also accompanied by CnH2n + and CnH2n-1 + ions in much less abundance. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 73. 73 Mass spectrum of dodecane C3H7 + C4H9+ C2H5 + C5H11+ C6H13+ C7 C8 C9 C10 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 74. Branched chain alkanes 74 Bond cleavage takes place preferably at the site of branching. Due to such cleavage, a more stable secondary or tertiary carbonium ions results. Generally, larger substituent at branch is eliminated readily as a radical. The radical achieves stability by the delocalization of lone electron. The relative abundance of the parent ion is least and is mostly not observed. Great number of fragments result from a branched chain compound compared to the straight chain compound. It is due to greater pathways available for cleavage. The signals corresponding CnH2n-1 + ions follow weak signals which appear 2 units below them. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 75. 75 Mass spectrum of 3,3 dimethyl hexane C2H5+ C3H7+ C4H9+ C5H11+ C6H13+ C7H15+ University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 76. Alkene 76 The molecular ion peak in the spectra of unsaturated compounds is more intense than the corresponding saturated analogues. The reason is the better resonance stabilization of the charge on the cation formed by the removal of one of the π electrons. Mono–olefines contain CnH2n-1 + ions in their mass spectra. The relative abundance of the molecular ion peak decreases with increasing molecular mass. A cyclic olefines also shows group of peaks which are 14 mass unit apart. The general mode of fragmentation induced by a double bond is the allylic cleavage. The CnH2n ions (fragments) formed by McLafferty rearrangement are more intense. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 77. Cycloalkanes 77 The relative abundance of the molecular ion of cycloalkane is more as compared to the corresponding alkane. It favors cleavage at the bond connecting the ring to the rest of the molecule. Fragmentation of the ring is usually characterized by the loss of two carbon atoms as C2H4 +(28 mass units) and C2H5 +(29 mass units). The stability of the fragment ion depends upon the size of the ring. Fragment ions are commonly observed by the loss of alkenes or alkenyl ions. The side chain on the ring breaks and lone or odd electron remains on the ring. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 78. 78 Mass spectrum of cyclohexane University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 79. 79 Mass spectrum of naphthalene University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 80. 80 Mass spectrum of P-Xylene University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 81. acetylenes 81 For 1-Butyne and 2-Butyne, the molecular peak is the base peak. The relative abundance of the molecular ion peak decreases as the molecular mass of the alkyne increases. In alkynes, the fragment ions are generally formed by the loss of alkyl radicals. Thus, M+-15, M+-29 etc. peaks are generally noticed in the mass spectra of alkynes. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 82. Aromatic compounds 82 The molecular ion peak in aromatic compounds is fairly abundant as compared to the corresponding alkanes and alkenes containing the same number of carbon atoms. In aromatic compounds, M++1 and M++2 are also noticed. The reason is fairly large abundance of the molecular ion peak. In case of polynuclear hydrocarbons, doubly or triply charged (M2+,M3+ ions) are possibly formed. Doubly charged molecular ions(m/2e)appear at integral m/e values. If the aromatic ring is substituted by an alkyl group, a prominent peak is formed at m/e 91.Hence benzyl (C6H5CH2 +) cation formed rearranges to tropylium cation.(C7H7 +) University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 83. 83 The benzyl cation formed rearranges to more stable tropylium cation which appears at m/e 91. Tropylium cation in turn loses a molecule of acetylene to form C5H5+ at m/e 65. Cleavage of a carbon-carbon bond which is in the β-position to the aromatic ring is an energetically favoured fragmentation mode. CH3 CH3 + + -(CH3CH2). Benzyl cation rearrangement m/e 91 m/e 65 loss of -C2H2 ]+. ]+. R + rearrangement m/e 91 ]+. M + (parent ion) tropylium cation University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 84. 84 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 85. Mass spectrum of toluene University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana 85
- 86. 86 The molecular ion peak of primary and secondary alcohol is usually of low abundance. It is not detected in tertiary alcohols. The parent ion peak is formed by the removal of one electron from the lone pairs on the oxygen atom of primary and secondary alcohols. The number of fragmentation modes in alcohols depend upon the fact whether it is primary, secondary or tertiary alcohol. The fragmentation of carbon-carbon bond adjacent to oxygen atom(α- cleavage) is the preferred fragmentation mode. Alcohols University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 87. 87 The signal at m/e 31 appears in large abundance in the mass spectrum of methanol and other aliphatic primary alcohols. The signal corresponds to the formation of oxonium ion ( CH2= OH+) and is formed by the cleavage of carbon-hydrogen bond in methanol. Primary alcohols show M+-18 peaks corresponding to the loss of water. C H3 OH C H2 O + H +. -H. R OH C H2 O + H -RCH2. +. M + . (Primary alcohol) m/e 31 m/e 31 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 88. 88 A primary alcohol having a chain of four or more carbon atoms shows a peak which corresponds to M+-(18-CnH2n). It can be shown mechanistically as follows: Long chain members may show peaks corresponding to successive loss of H- radicals at M-1,M-2,M-3. It can be represented as shown. H O C H3 C H3 H O H2 + C H3 CH3 + . + C H3 CH2 . + C H3 CH3 ]+. C H3 CH3 O H H C H3 O + H C H3 O H C H3 O + (M + .) M + -1 M + -2 M + -3 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 89. 89 The CH2=OH+ is the most significant peak in the spectra of primary alcohols. Secondary alcohols cleave to give prominent peaks due to R- CH=OH+ at m/e 45,59,73….Tertiary alcohols fragment to give prominent peaks due to RR’C=OH+ at m/e 59,73,87…. In addition to the α- cleavage, primary alcohols also undergo β-,γ-,δ- cleavage to form peaks at m/e 45,59, 73… University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 90. Mass spectrum of1-Butanol 90 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 91. Mass spectrum of2-butanol 91 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 92. 92 The relative abundance of the parent ion (M+) of aromatic alcohol is fairly large. Some of the fragment modes of benzyl alcohol are loss of one, two or three hydrogen atoms. The fragment ion, (M+-H) further eliminates CHO radical. (M+-H) fragment of benzyl alcohol also rearranges to form hydroxy tropylium ion. Aromatic alcohols University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 93. 93 The –OH group in the benzylic positions fragments in a way which favors retention on the aryl group. OH OH C H3 O H C H3 O + C6H5 + C4H3 + -[C2H2] -H. -CO + -H. Hydroxy tropylium ion -2H. (M + ) m/e 77 m/e 51 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 94. 94 Aliphatic ethers show molecular ion peak almost of negligible abundance. The presence of oxygen atom in ethers can be known from strong peaks at m/e 31,45, 59 etc. and these peaks represent RO+ and ROCH2+ fragments. The most characteristic fragmentation mode is the loss of one of the alkyl groups to form an oxonium ion (RO+) or the alkyl cation. The ions so formed is the result of α- cleavage. Ether University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 95. 95 Mass spectrum of Diethyl ether C H3 O CH3 C H3 O + CH3 C H2 O + H C H3 O + H m/e 45 m/e 31 m/e 59 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 96. Aryl ethers 96 In case of aromatic ethers, the molecular ion peak is fairly abundant. Methyl phenyl ethers show two main fragmentations. Primary fission occurs at the bond β- to the ring. Loss of methyl gives an ion M-15.It further splits to lose carbon monoxide. The fragmentation pattern is shown below: University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 97. 97 Mass spectrum of anisole O CH3 OH + -CH3. ]+. ]+ -CO m/e 93 M + . m/e 108 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 98. Aliphatic aldehydes and ketones 98 The intensity of the molecular ion peak decreases as the alkyl chain length increases. The major fragmentation processes are α and β – cleavage. In α- cleavage, the bigger group on either side of the carbonyl group (ketone ) preferably lost. In aldehydes and ketones containing γ- hydrogen atom, Mc Lafferty rearrangement ion is most significant. In an aldehyde, which is not α- substituted, a peak due to this is formed at m/e 44. it may be base peak. The Mc Lafferty rearrangement ion in methyl ketones which are not α- substituted appears at m/e 58 and is quite abundant. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 99. 99 In lower aldehydes, α-cleavage is prominent with retention of charge on oxygen. In aldehydes, methyl or alkyl radical is preferably lost compared to hydrogen radical. C H3 O H + C H3 O + C H3 O + H H O C H2 H OH + m/e 44 m/e 29 M + (m/e 72) (MR ion) Base peak University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 100. Mass spectrum of Pentanal 100 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 101. 101 In these compounds, parent ion peak is intense. M+.-1, M+.-28 due to the elimination of CO in benzaldehyde are formed. Peak at m/e 77 due to C6H5 + followed by the one at m/e 51 due to C4H3 + also result. Benzaldehyde Aromatic aldehydes and ketones H5C6 O + C6H5CHO + C6H6 + C6H5 + C4H3 + m/e 51 m/e 77 m/e 78 (M + .-1) (M + .) -(C2H2) -(H) -(CO) University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 102. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana 102 R O O + C4H3 + m/e 51 m/e 77 m/e 105 M + (parent ion) _R. -CO _C2H2 ]+. + In ketones, the loss of larger group is preferably by a α- cleavage. Consider the fragmentation of alkyl phenyl ketone:
- 103. Mass spectrum of Benzaldenyde University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana 103
- 104. Mass spectrum of Benzophenone 104 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 105. 105 The molecular ion peak in aliphatic acids is less intense as compared to that of aromatic acids. Carboxylic group is directly eliminated by α- cleavage and a signal is formed at m/e 45. If α- carbon atom is not substituted in aliphatic acids containing a γ- hydrogen atom, a Mc Lafferty rearrangement ion is formed at m/e 60. It is often the base peak. In short chain acids, M-OH+. And M-COOH+. Peaks are prominent. Aliphatic acids C H3 OH O OH O C H3 O + C H3 CH3 + m/e 43 m/e 71 m/e 45 (M + ) m /e 88 ]+ M - (OH) M + .-(COOH) University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 106. Mass spectrum of Pentanoic acid University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana 106 H OH O C H2 OH OH (M + ) (MR ion) (base peak) m/e 60 H OH O O OH C H2 OH CH3
- 107. Aromatic acids 107 In aromatic acids, the parent ion parent is intense. Some other prominent peaks are M-17+ and M-45+ If an alkyl group is present or any other hydrogen bearing group be present ortho to –COOH group, then a signal due to M-18 (loss of water molecule) is also observed. It is called ortho effect. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 108. Esters 108 The molecular ion peak is weak. The fragment ion due to α- cleavage is usually observed. In methyl esters, peaks due to R-CO+,R+,CH2O+ and CH3OCO+(m/e 59) are observed. The methyl esters not substituted at the α- carbon atom show McLafferty rearrangement ion at m/e 74. Methyl substitution at α-carbon atom shifts the position of McLafferty rearrangement peak at m/e 88. The molecular ion peak is comparatively more intense. Benzyl acetate and alkyl acetate eliminate neutral ketene molecule to form a base peak. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 109. 109 C H3 O CH3 O ]+. C H3 O O + C H3 CH3 OCH3 + H CH3 O C H2 O CH3 OH m/e 59 (M + - molecular ion) + m/e 43 m/e 31 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 110. Mass spectrum of Diethyl ether University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana 110
- 111. Amides 111 The molecular ion peak of straight chain monoamides is usually discernible. The McLafferty rearrangement peak in amides is usually the base peak. The M.R. ion appears at m/e 59.Primary amides give a strong peak at m/e 44 due to H2N-C=-O+ A moderate peak at m/e 86 results from γ-δ- carbon cleavage , possibly accompanied by cyclisation. When the N-alkyl groups on C2 are longer and acyl moiety is shorter than C3, another mode of cleavage predominates. This is the cleavage of the N-alky group beta to the nitrogen atom, and cleavage of the C-N bond with migration of α-H atom of the acyl moiety. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 112. Mass spectrum of Benzamide University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana 112
- 113. Halogen compounds 113 The molecular ion abundance of a particular alkyl halide increases as the electro negativity of the halogen substituent decreases. The relative abundance of the molecular ion decreases with increase in chain length and increasing in branching. Compounds containing chlorine and bromine show characteristic isotope peaks. A compound containing one chlorine atom shows M+2 peak which is one third in intensity of parent peak. A mono bromo compound shows (M+2) peak which is of the same intensity compared to the parent peak. In the parent ion, charge resides on the halogen atom. Important fragmentation mode is α- cleavage with charge retention by the halogen containing fragment. Another mode leads to the loss of halide radical. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 114. Mass spectrum of Ethyl chloride 114 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 115. Mass spectrum of Ethyl bromide 115 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 116. Mass spectrum of Dichloroethane 116 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 117. Mass spectrum of Dibromonitromethane 117 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 118. 118 If the molecular ion is formed at the odd mass number, then the molecule carries an odd number of nitrogen atoms. The molecular ion peak in monoamines is formed in very small abundance and is undetectable in long chain or branched chain amines. For primary amines, the base peak is formed at m/e 30 due to CH2=NH2 +.It results from the molecular ion by α- cleavage. The parent ion may lose an alkene to form a fragment ion at M+-CnH2n. Loss of largest branch from the α- carbon atom is preferred. Amines University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 119. 119 In higher aliphatic alkyl amines, β- cleavage is not very significant.γ- cleavage is sometimes preferred. N R R 1 R 2 R 4 R 3 N + C H3 C H3 CH3 CH3 N C H3 C H3 CH3 CH3 -[R 2 ] +. here R 2 . R 1 , or R University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 120. 120 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 121. 121 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 122. Nitro compounds 122 The molecular ion peak in aliphatic nitro compounds is usually absent but it is prominent in aromatic compounds. In aliphatic compounds, the signals due to NO+ and NO2 + are usually observed. In aromatic nitro compounds, the signals for NO+ and NO2 + are commonly observed. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 123. Mass spectrum of 1-Chloro-3- nitrobenzene University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana 123
- 124. Aliphatic nitriles 124 The molecular ion peak of aliphatic nitriles is weak or may be absent. A weak but diagnostically useful (M_1) peak if formed by the loss a α- hydrogen atom to form a stable ion. The base peak of straight chain nitriles between C4 and C9 is m/e 41.This peak is due to the ion resulting from hydrogen rearrangement from hydrogen rearrangement in a six membered transition state. University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 125. READING A MASS SPEC 125 Fragment Due to loss of… Interpretation M+• -1 -H• Aldehydes, tert. Alcohols, cyclic amines M+• -2 Multiple -H• Secondary alcohols M+• -3 Multiple -H• Primary alcohols M+• -4 to -13 (doubtful) Consider contaminants M+• -14 (doubtful) CH2• , N• not good losses M+• -15 CH3• Available methyl groups, methylesters M+• -16 O• Peroxides M+• -17 OH• Alcohols, phenols, RCO2H M+• -18 H2O alcohols M+• -19 -F• M+• -20 -HF M+• -21 to -25 No peaks expected M+• -26 HCCH M+• -27 •HC=CH2 or HCN HCN from pyridine, anilines M+• -28 CO or CH2=CH2 Check for McLafferty University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana
- 126. 126 University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana