This document provides standard operating procedures for analyzing various water quality parameters such as total hardness, pH, conductivity, alkalinity, and silica. The methods include titrating water samples against standard solutions of EDTA, H2SO4, and other reagents to determine concentrations. Precise procedures for sample collection, preparation, and use of analytical equipment like pH meters are outlined.



![GURU GOBIND SINGH REFINERY LIMITED,
BATHINDA
WATER BLOCK UNIT
STANDARD OPERATING PROCEDURE FOR
WATER ANALYSIS
Issue
No.
01
Issue Date
11.01.2018
Revision Revision Date Clause
Page 4 of 57
Signature: Signature by: Signature:
Prepared by: Pushpendra Reviewed by: Approved by :
Designation: Lab Designation: Designation:
PURPOSE : To determine the pH of water
SCOPE : All types of water
RESPONSIBILITY : Chemist
DESCRIPTION : As follows
pH measurement plays a very important role in the water chemistry. pH is Defined as the
negative logarithm of the hydrogen ion concentration (to the Base 10)
pH = log10[H +
]
pH range is 0-14. An acidic solution is the one in which pH is < 7 where an
alkaline solution is the one in which pH is > 7. A neutral solution has a pH of 7.
In a neutral solution the hydrogen ion & hydroxide ion concentrations are equal
& each corresponds to 107
moles/litre.
pH MEASUREMENT
USE OF pH METER: -
An accurate pH measurement is possible with the help of pH meter.
A general Procedure for pH measurement using any standard pH meter](https://image.slidesharecdn.com/sop-wateranalysis-2-180801050357/85/Sop-water-analysis-2-4-320.jpg)



















![GURU GOBIND SINGH REFINERY LIMITED,
BATHINDA
WATER BLOCK UNIT
STANDARD OPERATING PROCEDURE FOR
WATER ANALYSIS
Issue
No.
01
Issue Date
11.01.2018
Revision Revision Date Clause
Page 32 of 57
Signature: Signature by: Signature:
Prepared by: Pushpendra Reviewed by: Approved by :
Designation: Lab Designation: Designation:
PURPOSE : To determine Dissolved Oxygen
SCOPE : All types of water
RESPONSIBILITY : Chemist
DESCRIPTION : As follows
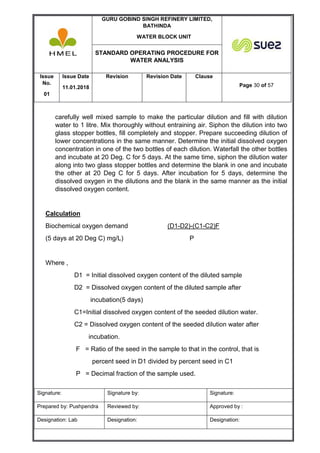
METHOD:
The dissolved oxygen in the sample oxidizes manganous hydroxide to manganic
hydroxide, which, in turn, oxidizes iodide to free iodine in an acid medium. The
iodine liberated is determined by titration.
REAGENT REQUIRED:]
1. Manganous sulfate solution: - Dissolve 480gm of manganous sulfate
(MnSO4.4H2O) in distilled water, filter and dilute to 1 litre. The Solution
should liberate not more than a trace of iodine when added to an acidified
solution of potassium iodide.](https://image.slidesharecdn.com/sop-wateranalysis-2-180801050357/85/Sop-water-analysis-2-32-320.jpg)



![GURU GOBIND SINGH REFINERY LIMITED,
BATHINDA
WATER BLOCK UNIT
STANDARD OPERATING PROCEDURE FOR
WATER ANALYSIS
Issue
No.
01
Issue Date
11.01.2018
Revision Revision Date Clause
Page 37 of 57
Signature: Signature by: Signature:
Prepared by: Pushpendra Reviewed by: Approved by :
Designation: Lab Designation: Designation:
water , presence of organic matter affect the solids contents drastically thus giving
misleading results.
SUMMARY OF METHOD:
A well mixed sample is evaporated to dryness in in an oven at 1030
C—1050
C to a
constant weight . The increase in weight represents the solid content.
PROCEDURE
(A) TOTAL SOLIDS (AT 1050
C)
1) Take out a known volume of well mixed water sample (unfiltered , as is water
sample) in a clean ,preweighed dish.
2) Choose a sample volume that will yield minimum residue of 50 mg to 250 mg. If
necessary & successive portions of sample to the same dish.
3) Evaporate to dryness on a steam bath or in drying furnace oven. Avoid splashing
while evaporating.
4) Dry the evaporated sample for at least 1 hour at 1030
C—1050
C.
5) Cool the dish in a desiccators to room temperature & weigh .
6) Repeat the cycle of drying , cooling & weighing until a constant weigh is obtained ,
Note the weight of residue .]
Total Solids = (A-B) X 1000](https://image.slidesharecdn.com/sop-wateranalysis-2-180801050357/85/Sop-water-analysis-2-37-320.jpg)