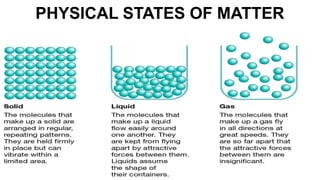
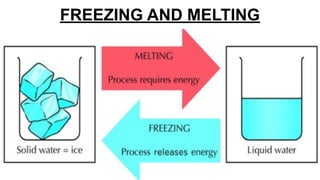
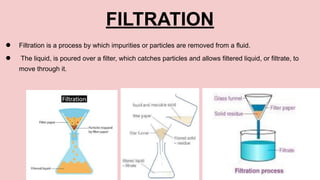
This document discusses different states of matter and properties. It defines mass and explains that both air and water have mass, though air is less dense. It describes the physical states of matter as solid, liquid or gas. Freezing and melting are defined as reversible changes between solids and liquids that involve temperature changes at specific freezing and melting points. Irreversible changes like burning cannot be reversed. Mixtures can be separated using sieves, strainers or filtration, while dissolving solids in liquids forms solutions from which the solids cannot later be separated by filtration.