Solid, Liquid, and Gas.ppt
•Download as PPT, PDF•
0 likes•4 views
This document discusses the properties of solids, liquids, and gases. It explains that in solids, particles are fixed in place with strong intermolecular forces, while in liquids they are closer together but with more motion. In gases, particles are far apart with minimal intermolecular forces and take the shape and volume of their container. It also describes evaporation as a process where molecules gain energy to break intermolecular bonds and become a gas, and condensation as the opposite where gases release energy and form bonds to become liquids. Dynamic equilibrium is reached when the rates of evaporation and condensation are equal in a closed system.
Report
Share
Report
Share
Recommended
More Related Content
Similar to Solid, Liquid, and Gas.ppt
Similar to Solid, Liquid, and Gas.ppt (20)
Topic 1 Mass relationship in chemistry (2021.01.14) (Updated).pdf

Topic 1 Mass relationship in chemistry (2021.01.14) (Updated).pdf
More from PoojaTripathi92
More from PoojaTripathi92 (10)
Recently uploaded
https://app.box.com/s/tkvuef7ygq0mecwlj72eucr4g9d3ljcs50 ĐỀ LUYỆN THI IOE LỚP 9 - NĂM HỌC 2022-2023 (CÓ LINK HÌNH, FILE AUDIO VÀ ĐÁ...

50 ĐỀ LUYỆN THI IOE LỚP 9 - NĂM HỌC 2022-2023 (CÓ LINK HÌNH, FILE AUDIO VÀ ĐÁ...Nguyen Thanh Tu Collection
This PowerPoint presentation, titled "Research Methods in Psychology for Cambridge AS Level Students," provides a comprehensive overview of essential research methodologies in psychology. It covers fundamental concepts such as experimental, correlational, and observational methods, highlighting their advantages and limitations. The presentation delves into the design of experiments, including independent and dependent variables, control groups, and random assignment. It also addresses ethical considerations, data collection techniques, and statistical analysis. Emphasizing practical application, the presentation includes examples of classic psychological studies and offers tips for designing and conducting research projects. It concludes with a discussion on interpreting results and the importance of critical evaluation, preparing students for both theoretical understanding and practical application in their AS Level psychology coursework.Research Methods in Psychology | Cambridge AS Level | Cambridge Assessment In...

Research Methods in Psychology | Cambridge AS Level | Cambridge Assessment In...Abhinav Gaur Kaptaan
This presentation was provided by William Mattingly of the Smithsonian Institution, during the closing segment of the NISO training series "AI & Prompt Design." Session Eight: Limitations and Potential Solutions, was held on May 23, 2024.Mattingly "AI & Prompt Design: Limitations and Solutions with LLMs"

Mattingly "AI & Prompt Design: Limitations and Solutions with LLMs"National Information Standards Organization (NISO)
Recently uploaded (20)
50 ĐỀ LUYỆN THI IOE LỚP 9 - NĂM HỌC 2022-2023 (CÓ LINK HÌNH, FILE AUDIO VÀ ĐÁ...

50 ĐỀ LUYỆN THI IOE LỚP 9 - NĂM HỌC 2022-2023 (CÓ LINK HÌNH, FILE AUDIO VÀ ĐÁ...
Keeping Your Information Safe with Centralized Security Services

Keeping Your Information Safe with Centralized Security Services
Jose-Rizal-and-Philippine-Nationalism-National-Symbol-2.pptx

Jose-Rizal-and-Philippine-Nationalism-National-Symbol-2.pptx
The impact of social media on mental health and well-being has been a topic o...

The impact of social media on mental health and well-being has been a topic o...
slides CapTechTalks Webinar May 2024 Alexander Perry.pptx

slides CapTechTalks Webinar May 2024 Alexander Perry.pptx
Basic Civil Engineering Notes of Chapter-6, Topic- Ecosystem, Biodiversity G...

Basic Civil Engineering Notes of Chapter-6, Topic- Ecosystem, Biodiversity G...
The Art Pastor's Guide to Sabbath | Steve Thomason

The Art Pastor's Guide to Sabbath | Steve Thomason
Benefits and Challenges of Using Open Educational Resources

Benefits and Challenges of Using Open Educational Resources
Pragya Champions Chalice 2024 Prelims & Finals Q/A set, General Quiz

Pragya Champions Chalice 2024 Prelims & Finals Q/A set, General Quiz
Research Methods in Psychology | Cambridge AS Level | Cambridge Assessment In...

Research Methods in Psychology | Cambridge AS Level | Cambridge Assessment In...
Matatag-Curriculum and the 21st Century Skills Presentation.pptx

Matatag-Curriculum and the 21st Century Skills Presentation.pptx
Telling Your Story_ Simple Steps to Build Your Nonprofit's Brand Webinar.pdf

Telling Your Story_ Simple Steps to Build Your Nonprofit's Brand Webinar.pdf
Mattingly "AI & Prompt Design: Limitations and Solutions with LLMs"

Mattingly "AI & Prompt Design: Limitations and Solutions with LLMs"
Solid, Liquid, and Gas.ppt
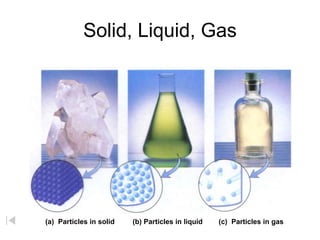
- 1. Solid, Liquid, Gas (a) Particles in solid (b) Particles in liquid (c) Particles in gas
- 2. Solid H2O(s) Ice Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 31
- 3. Ice H2O(s) Ice Photograph of ice model Photograph of snowflakes Copyright © 2007 Pearson Benjamin Cummings. All rights reserved.
- 4. Liquid H2O(l) Water Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 31 In a liquid • molecules are in constant motion • there are appreciable intermolecular forces • molecules are close together • Liquids are almost incompressible • Liquids do not fill the container
- 5. Gas H2O(g) Steam Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 31
- 6. Liquids The two key properties we need to describe are EVAPORATION and its opposite CONDENSATION add energy and break intermolecular bonds EVAPORATION release energy and form intermolecular bonds CONDENSATION
- 8. Gas, Liquid, and Solid Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 441 Gas Liquid Solid
- 9. States of Matter Solid Liquid Gas Holds Shape Fixed Volume Shape of Container Free Surface Fixed Volume Shape of Container Volume of Container heat heat
- 10. Some Properties of Solids, Liquids, and Gases Property Solid Liquid Gas Shape Has definite shape Takes the shape of Takes the shape the container of its container Volume Has a definite volume Has a definite volume Fills the volume of the container Arrangement of Fixed, very close Random, close Random, far apart Particles Interactions between Very strong Strong Essentially none particles
- 11. • To evaporate, molecules must have sufficient energy to break IM forces. • Molecules at the surface break away and become gas. • Only those with enough KE escape. • Breaking IM forces requires energy. The process of evaporation is endothermic. • Evaporation is a cooling process. • It requires heat. Evaporation
- 12. Change from gas to liquid Achieves a dynamic equilibrium with vaporization in a closed system. What is a closed system? A closed system means matter can’t go in or out. (put a cork in it) What the heck is a “dynamic equilibrium?” Condensation
- 13. When first sealed, the molecules gradually escape the surface of the liquid. As the molecules build up above the liquid - some condense back to a liquid. The rate at which the molecules evaporate and condense are equal. Dynamic Equilibrium
- 14. As time goes by the rate of vaporization remains constant but the rate of condensation increases because there are more molecules to condense. Equilibrium is reached when: Rate of Vaporization = Rate of Condensation Molecules are constantly changing phase “dynamic” The total amount of liquid and vapor remains constant “equilibrium” Dynamic Equilibrium
- 15. • Vaporization is an endothermic process - it requires heat. • Energy is required to overcome intermolecular forces • Responsible for cool earth • Why we sweat Vaporization
- 16. Energy Changes Accompanying Phase Changes Solid Liquid Gas Melting Freezing Deposition Condensation Vaporization Sublimation Energy of system Brown, LeMay, Bursten, Chemistry 2000, page 405