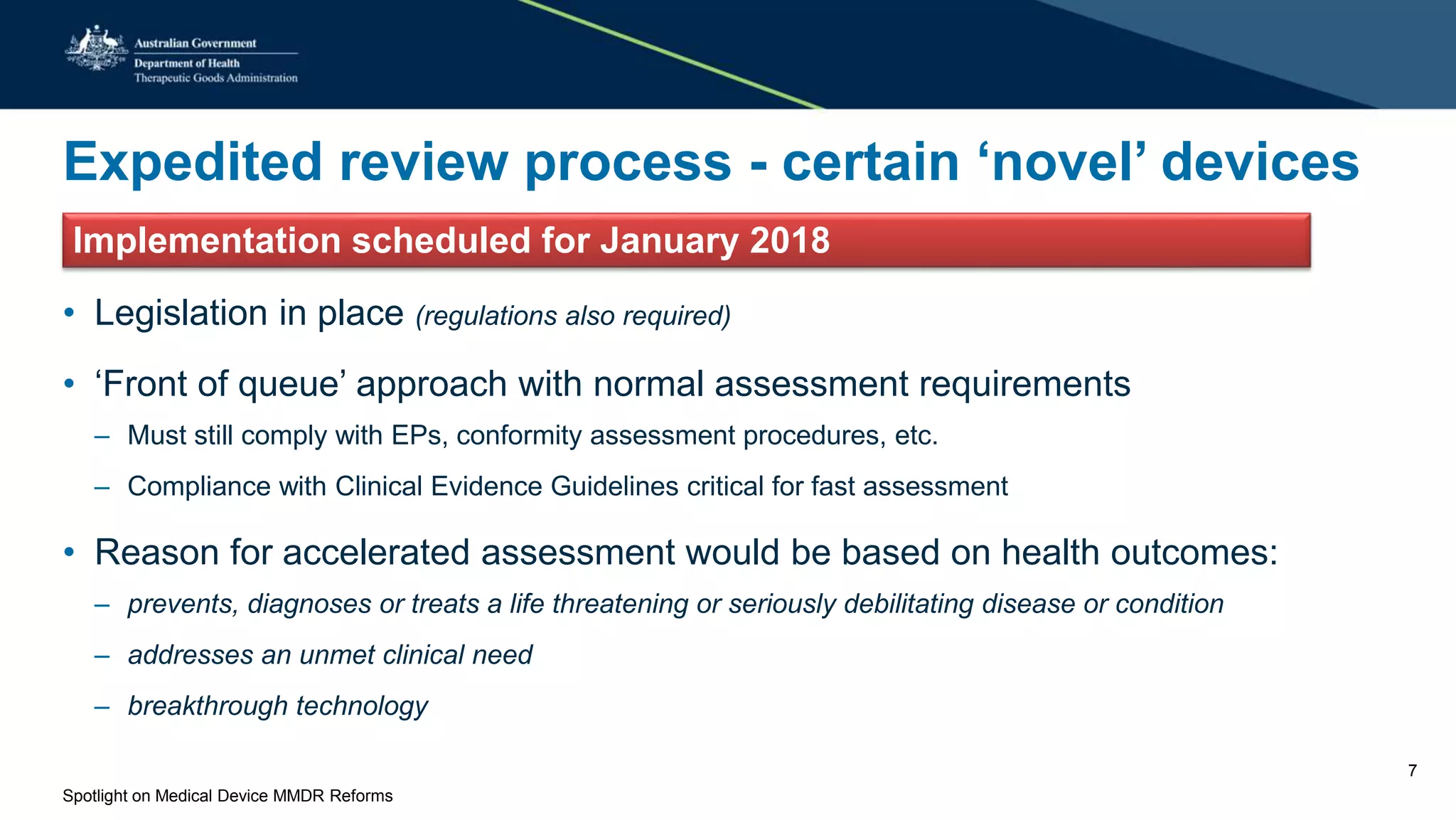
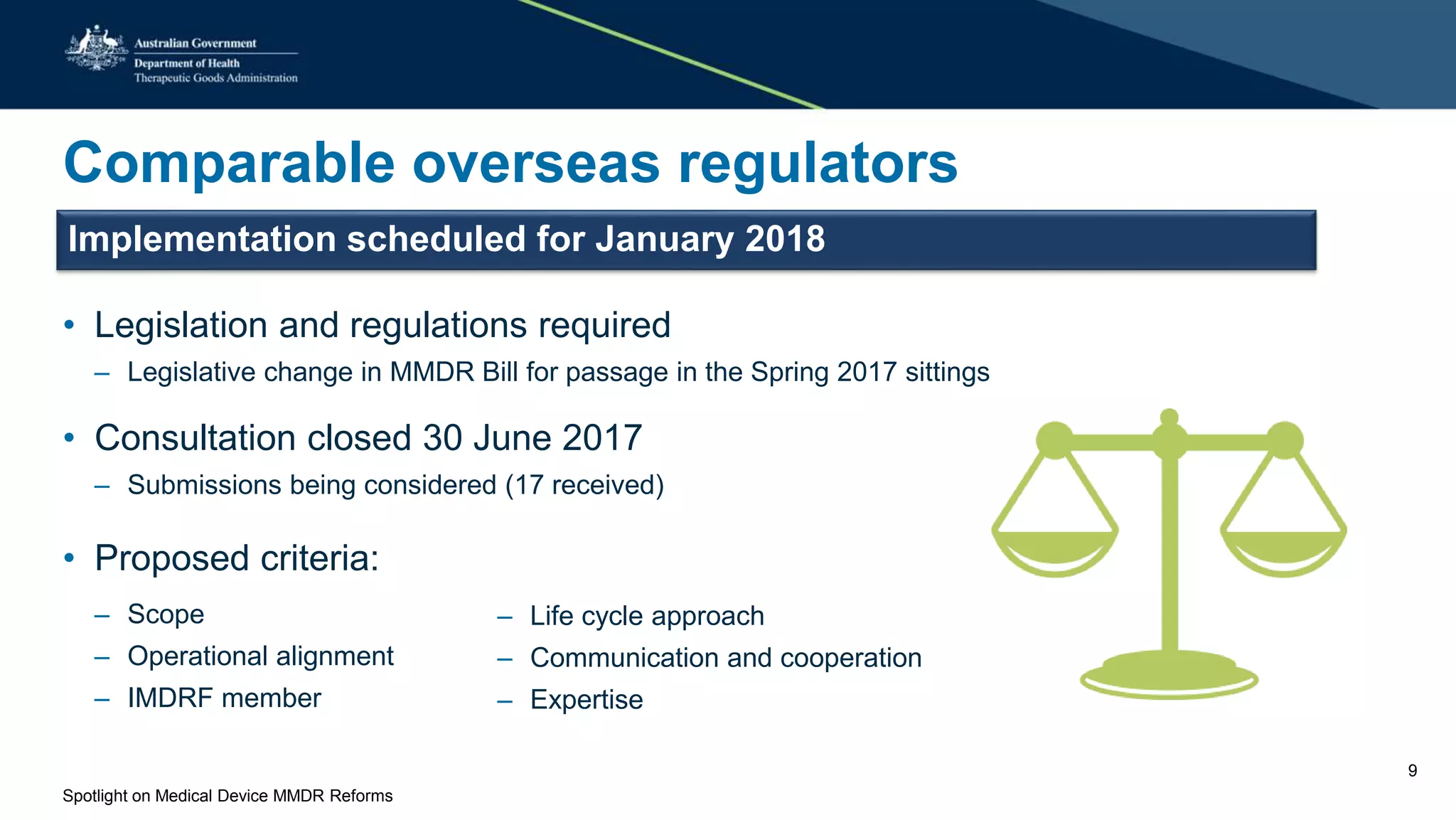
The document discusses reforms to the regulation of medical devices in Australia, highlighted by the government's acceptance of 56 out of 58 recommendations from a comprehensive review. Key initiatives include the establishment of conformity assessment bodies, an expedited review process for novel devices, alignment with European regulations, and enhanced post-market monitoring systems. Upcoming legislative changes and consultations are planned to implement these reforms by January 2018.