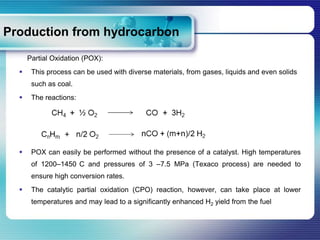
Hydrogen can be produced through various processes including steam methane reforming, partial oxidation, coal gasification, water electrolysis, and photolysis. Steam methane reforming is the most efficient current method using natural gas as a feedstock. It involves catalytic reforming of methane with steam at high temperatures. Partial oxidation and coal gasification are other thermal processes that can use diverse carbon-containing feedstocks to produce hydrogen and carbon monoxide through partial combustion reactions. Water electrolysis involves passing an electric current through water to dissociate it into hydrogen and oxygen gas. Alkaline electrolysis is a mature technology while PEM electrolysis offers advantages like easier construction and higher purity products. Photolysis uses solar energy to split water directly into hydrogen






![Alkaline electrolysis
- Alkaline electrolyte electrolyzers represent a
very mature technology that is the current
standard for large-scale electrolysis.
Common electrolyte: aqueous potassium
hydroxide (KOH) at 30% concentration
Operation Conditions: 70-100oC and 1- 30bar
Operational voltage: 1.7-2.2 V
Current density: 0.2-0.6 A/cm2
Electricity Consumption: 4.2 – 5.6 kWh/Nm3
Can utilize cost effective electrode
materialsDiaphragm often asbestos
Efficiency: 70-80% (based on hydrogen HHV) [1]
Russell H. Jones & George J. Thomas, “Materials for the
Hydrogen economy”, 2008, p.40](https://image.slidesharecdn.com/slidenhinliusch-130406095506-phpapp01/85/Slide-nhien-li-u-s-ch-8-320.jpg)
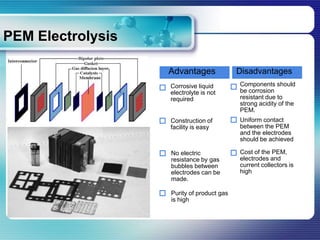
![PEM Electrolysis [1]
Polymer electrolyte water
Operational principle electrolysis (PEWE) uses a
The water flows from the plate to the polymer electrolyte membrane as
anode through the current collector, and a medium of ion transfer instead of
reacts to make protons. solution electrolyte in AWE. This
Current collectors are porous conductors method is often called polymer
that allow electrons to transfer from electrolyte membrane or proton
electrode to outer circuit and allow reactant exchange membrane (PEM) water
gas from bipolar plate to electrode. electrolysis, too.
The protons are transported through the
PEM to cathode side, and hydrogen is
generated at the cathode.
The PEM also works as a separator of
product gases.
[1] Seiji Kasahara et al., “Water electrolysis” in
“ Nuclear hydrogen production handbook”, 2011](https://image.slidesharecdn.com/slidenhinliusch-130406095506-phpapp01/85/Slide-nhien-li-u-s-ch-9-320.jpg)
![Steam electrolysis[1]
The process of the high-temperature electrolysis (HTE) of steam is a reverse reaction of the
solid-oxide fuel cell (SOFC): an oxygen ionic conductor is usually used as a solid-oxide
electrolyte.
The electrical energy demand, ΔG, decreases with increasing temperature. The ratio of ΔG to
ΔH is about 93% at 100 C and about 70% at 1000 C
An assembly unit consisting of 15 cells
Outer diameter: 12mm
Active area: 75 cm2
Hydrogen production rate: 100 NL/h.
Operation Conditions: 800oC
Operational voltage: 1.3 V
Current density: 0.45 A/cm2
[1] Seiji Kasahara et al., “Steam electrolysis” in
“ Nuclear hydrogen production handbook”, 2011](https://image.slidesharecdn.com/slidenhinliusch-130406095506-phpapp01/85/Slide-nhien-li-u-s-ch-11-320.jpg)
![Photoelectrolysis
Photoelectrolysis involves splitting water directly into hydrogen (H2) and oxygen (O2) using the
energy of sunlight.
The reactive decomposition occurs at 1.23 V, so the minimum bandgap for successful water
splitting is 1.23 eV, corresponding to light of 1008nm. [2]
Operational principle [3]
TiO2 electrode electrowas irradiated with light
consisting of wavelengths shorter than 415 nm (3.0
eV), photocurrent flowed from the Pt electrode to the
TiO2 de through the external circuit.
The direction of the current revealed that the
oxygen occurs at the TiO2 electrode and the
hydrogen occurs at the Pt electrode.
This observation shows that water can be
decomposed, using UV light, without the application
of an external voltage.](https://image.slidesharecdn.com/slidenhinliusch-130406095506-phpapp01/85/Slide-nhien-li-u-s-ch-12-320.jpg)



















