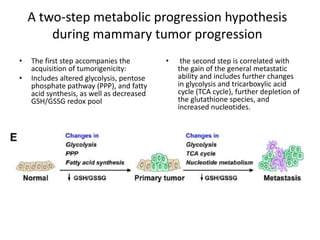
1. Cancer cells rely heavily on aerobic glycolysis, known as the Warburg effect, which produces less ATP than oxidative phosphorylation but supports rapid cell growth and proliferation.
2. Understanding the Warburg effect could allow researchers to stage and fingerprint tumors based on their metabolic profiles and capacities for invasiveness and proliferation.
3. Targeting metabolic pathways that support biomass production and interfering with lactate-based metabolic symbiosis between tumor cells and their microenvironment may provide approaches for combination anti-cancer therapies based on the Warburg effect.