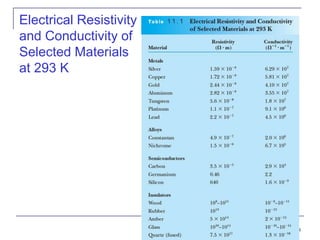
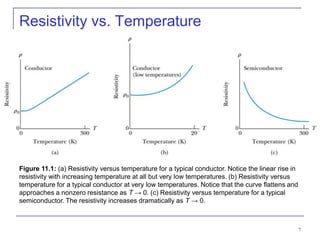
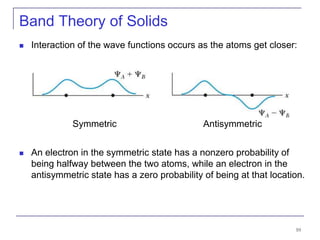
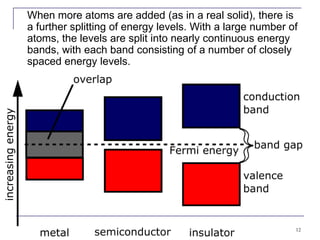
This document provides an overview of semiconductor theory and band theory of solids. It discusses how band theory can explain the differing electrical properties of conductors, semiconductors, and insulators based on their band structure and energy gaps. Semiconductors have a small energy gap between the valence and conduction bands that can be overcome by thermal excitation, allowing some electrons to reach the conduction band. Doping semiconductors with impurities can create n-type or p-type materials by introducing extra electrons or holes that increase conductivity. The document also covers thermoelectric effects in semiconductors.