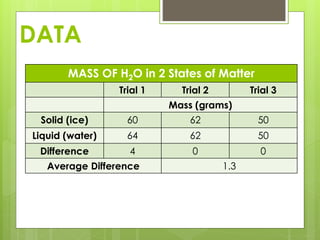
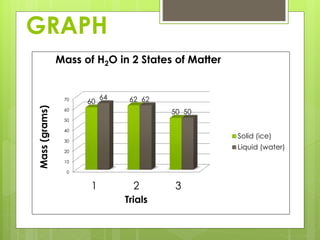
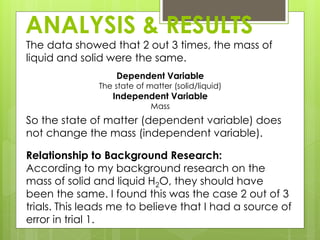
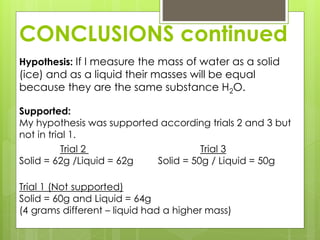
The student conducted an experiment to determine if water has the same mass in liquid and solid form. They weighed ice cubes and then melted the ice and weighed the liquid water. In two of the three trials, the masses were equal, supporting the hypothesis. However, in one trial the liquid water had a slightly higher mass, possibly due to sources of error like not fully drying the beaker. The student concluded that while the masses are nearly equal, more trials could provide more definitive evidence.