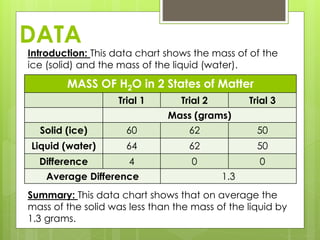
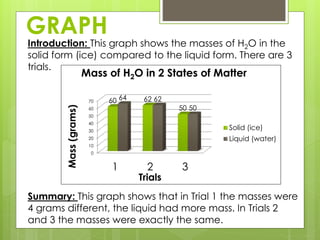
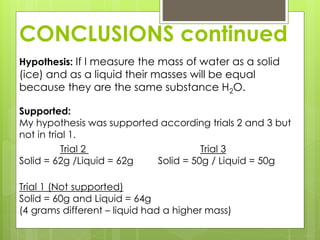
This lab report compares the mass of water in its solid (ice) and liquid states. The hypothesis is that the mass will be equal since it is the same substance, H2O, in different states. Data from trials show the mass was equal in the solid and liquid states for two trials but different in one trial, possibly due to error. The conclusion is that the mass of H2O is nearly the same in both solid and liquid forms.