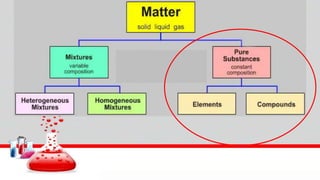
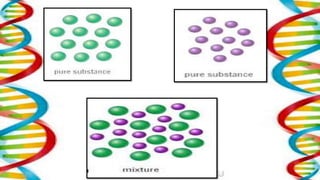
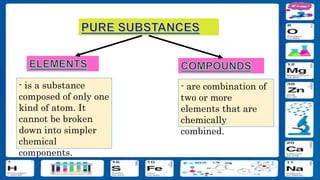
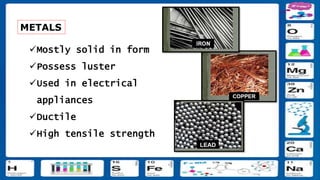
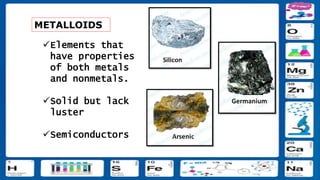
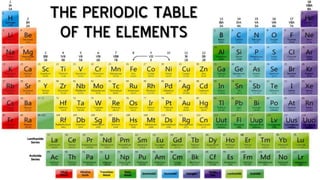
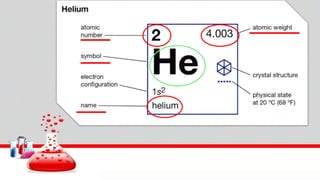
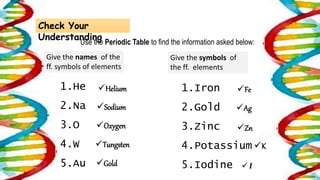
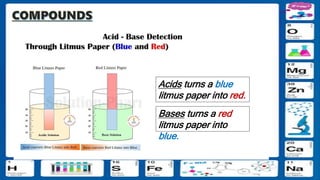
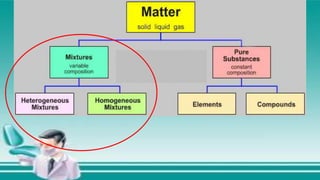
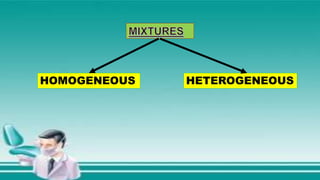
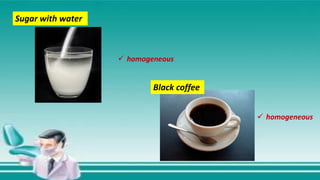
This document contains the rules and content for an online chemistry class. It begins with an opening prayer, then lists the rules for online class participation which include dressing appropriately, avoiding unrelated activities, being responsive, and muting microphones when not speaking. The document then outlines the units and lessons which cover matter, pure substances like elements and compounds, and mixtures including homogeneous and heterogeneous mixtures. Examples and assignments are provided throughout.