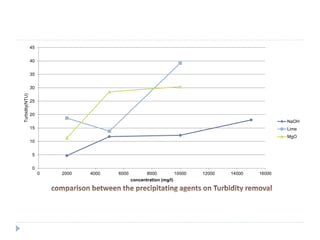
This presentation summarizes a study on the efficiency of different alkalis in removing pollutants from chrome tanning liquor waste in tannery industries in Bangladesh. The study characterized waste from two tanneries and tested the removal efficiency of chromium, COD, color, and turbidity using various alkalis including sodium hydroxide, lime, and magnesium oxide. Sodium hydroxide was found to have the fastest settling time but lime achieved the highest color removal. Magnesium oxide produced the least amount of sludge. The study concluded that chemical treatment alone was not sufficient and effluent should undergo further biological treatment. It also found that using mixtures of alkalis could increase cost efficiency for treatment.