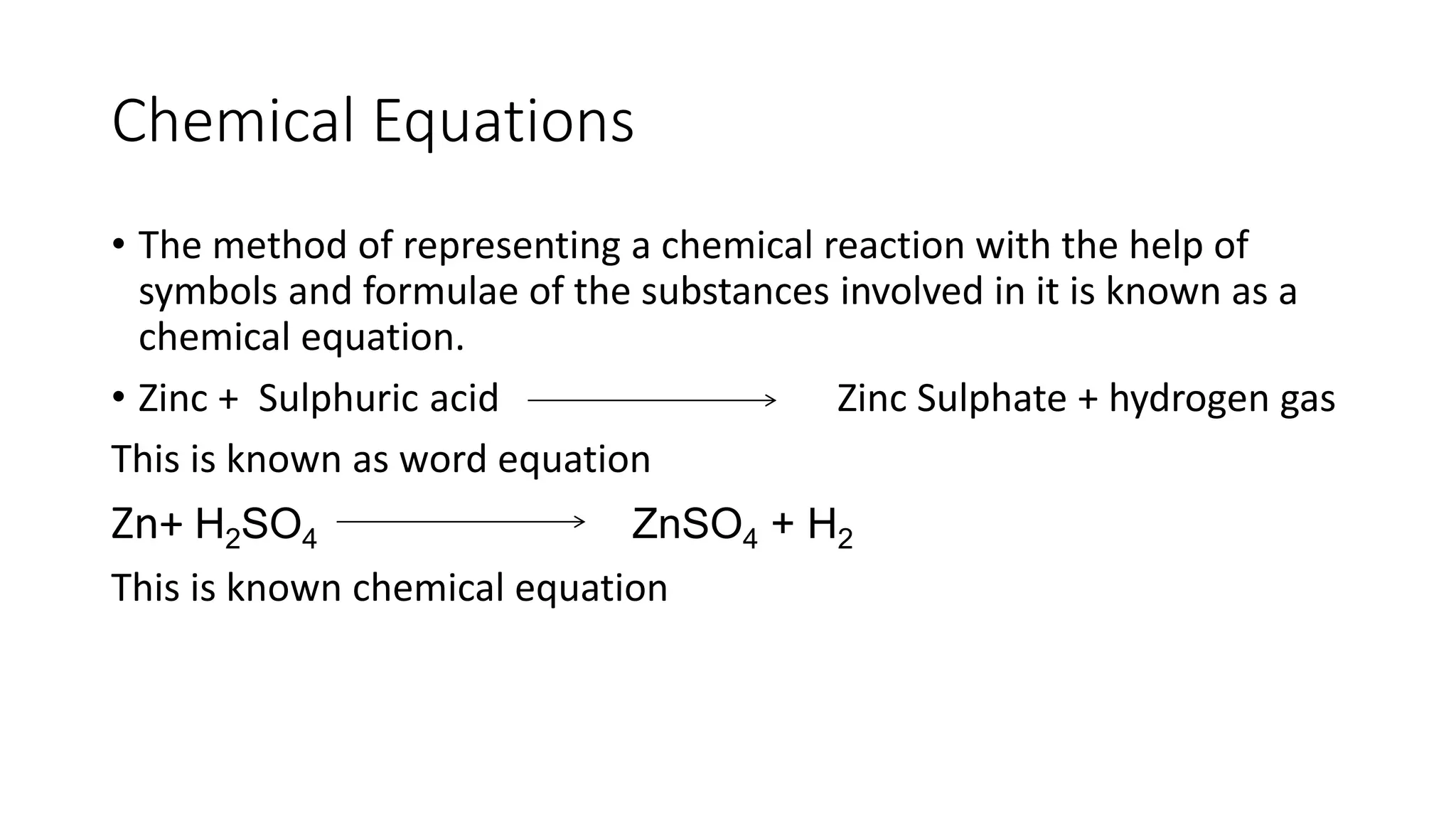
This document discusses chemical reactions and equations. It defines a chemical reaction as the transformation of one or more substances into new substances through bond breaking and forming. Reactants are the original substances, while products are the new substances formed. Chemical equations represent reactions symbolically, with balanced equations showing equal numbers of each type of atom on both sides. The document describes several types of chemical reactions including combination, decomposition, displacement, double displacement, and oxidation-reduction reactions. It provides examples to illustrate characteristic properties of reactions like gas evolution, precipitation, temperature change, and state changes. Oxidation and reduction reactions are explained along with examples of their effects in corrosion and food rancidity.