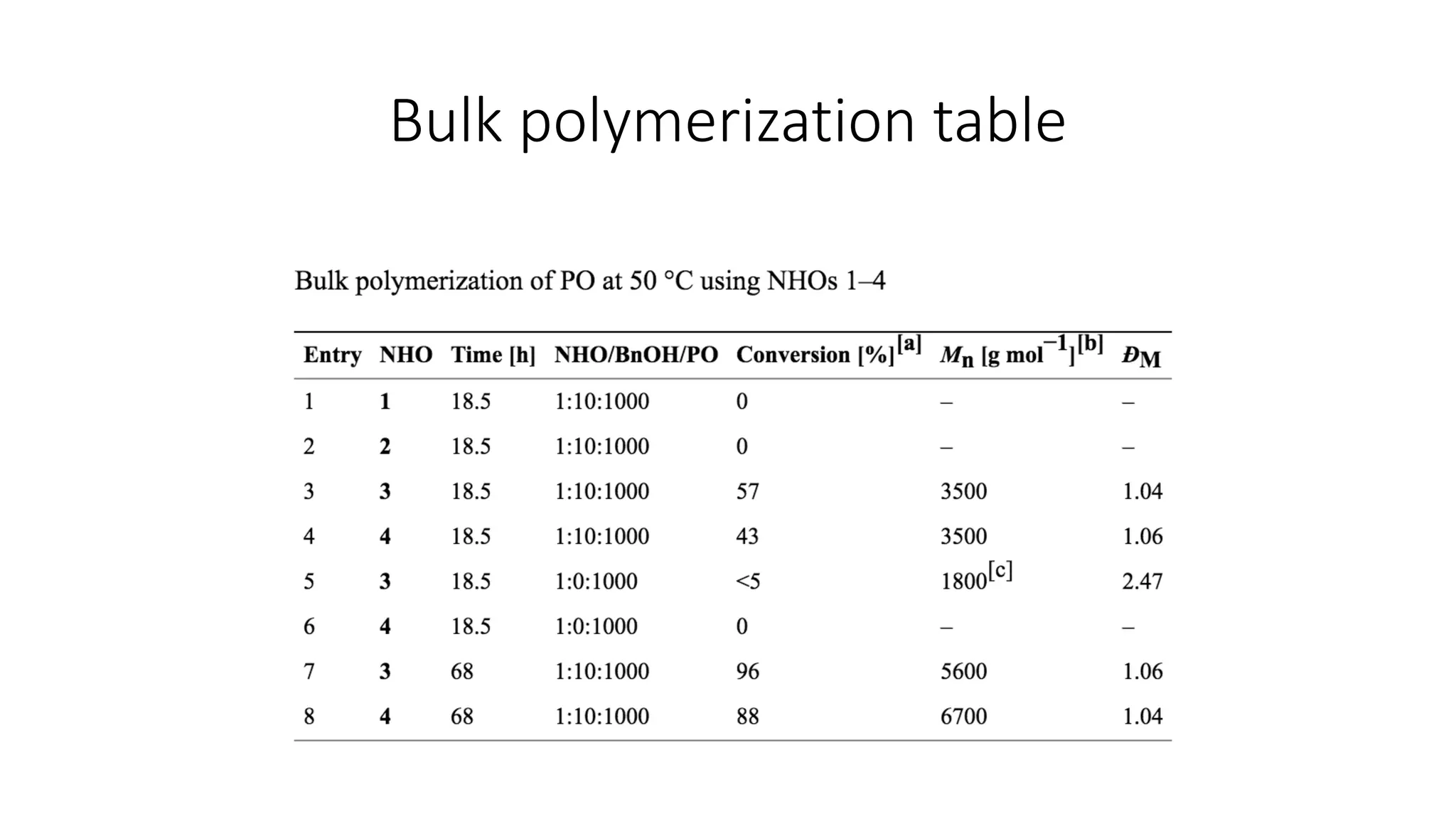
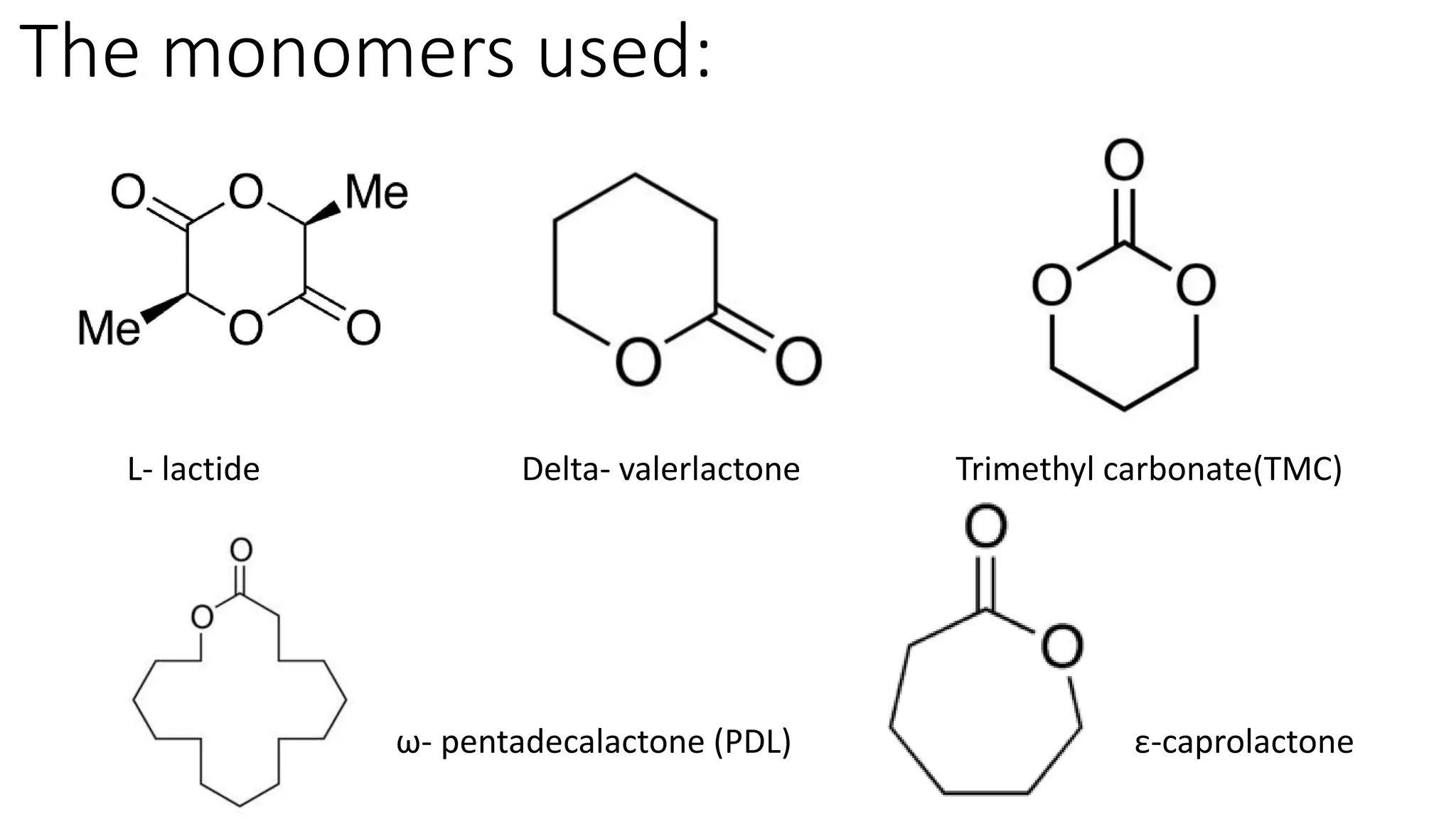
This document discusses the use of n-heterocyclic olefins as organic catalysts in ring opening polymerization, focusing on their advantages and the mechanisms involved. It highlights two papers that detail the properties and performance of various catalysts with respect to the polymerization of different monomers. Key findings include the effectiveness and stability of these catalysts, their lower toxicity compared to organometallic compounds, and their ability to produce high conversion rates.