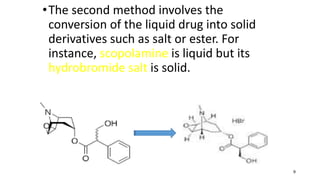
The document discusses key aspects of preformulation, which is the first step in developing a dosage form for a new drug substance. Some key points covered include:
- Preformulation determines important physicochemical properties of the drug that can influence dosage form development.
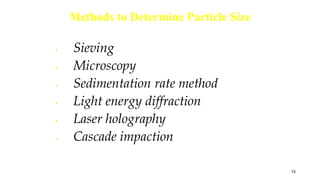
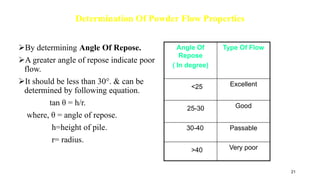
- Properties studied include physical description, melting point, organoleptic properties, particle size, and powder flow properties.
- The goals are to establish parameters needed for formulating an optimal drug delivery system and to determine compatibility with excipients.