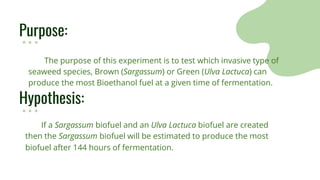
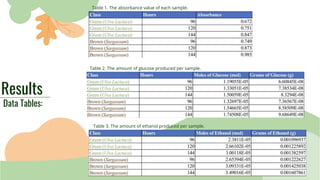
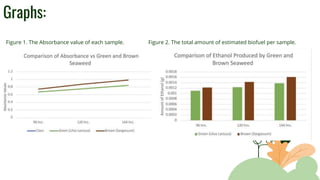
The purpose of the experiment was to determine which invasive seaweed, brown Sargassum or green Ulva Lactuca, could produce more bioethanol fuel over time. It was hypothesized that Sargassum would produce the most fuel after 144 hours of fermentation. Both seaweeds were processed and fermented for 96, 120, and 144 hours. Absorbance values indicated more glucose and ethanol were produced from Sargassum over time, supporting the hypothesis. While an estimation, more biofuel was calculated from Sargassum, showing potential practical use in reducing pollution from seaweed blooms. Limitations included ethanol amounts being estimates and seaweed composition varying by collection time.