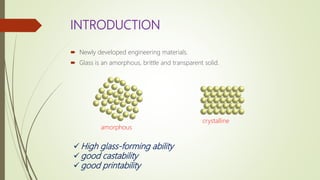
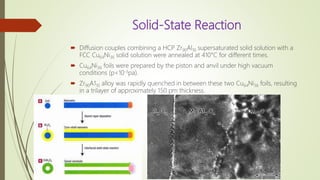
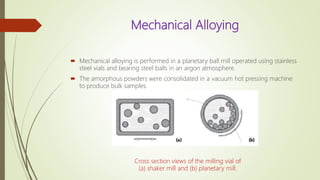
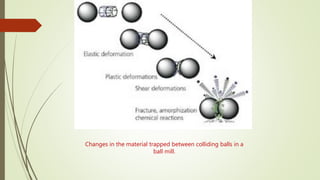
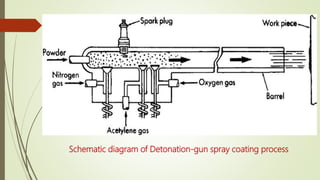
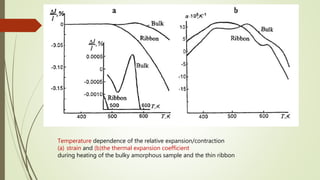
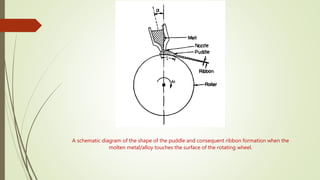
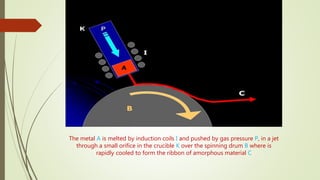
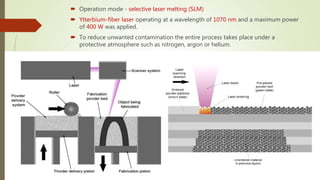
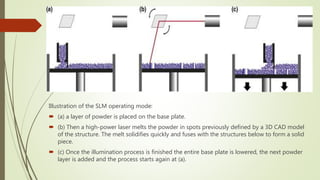
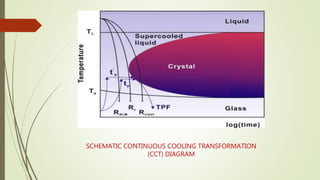
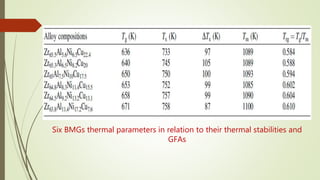
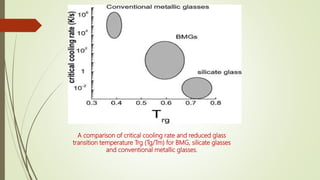
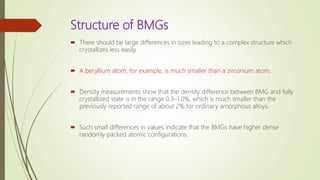
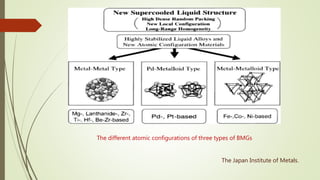
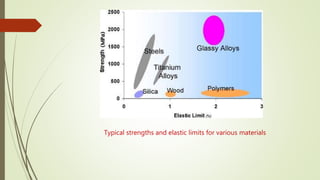
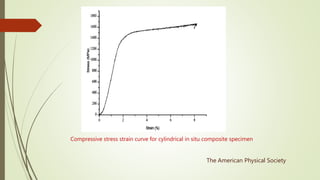
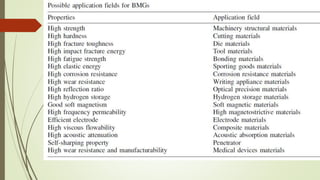
The document discusses bulk metallic glasses (BMGs), detailing their properties, preparation methods, and applications. BMGs, composed of amorphous alloys, exhibit unique characteristics such as high strength, ductility, and corrosion resistance, making them suitable for various engineering applications. Historical milestones in the development of BMGs and different processing techniques like melt-spinning and selective laser melting are also highlighted.