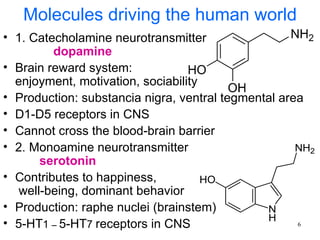
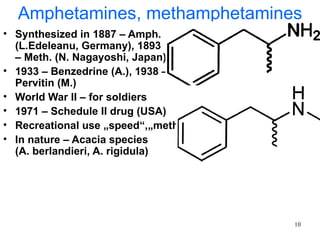
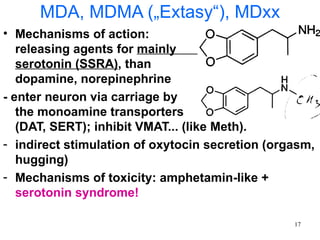
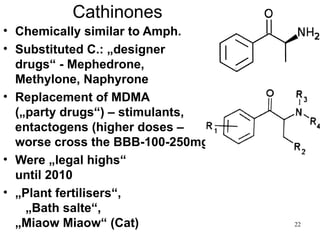
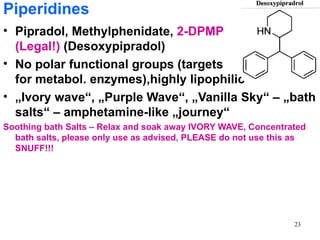
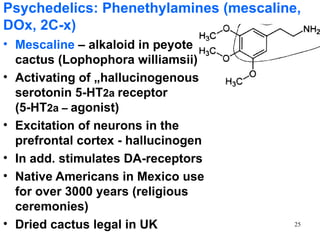
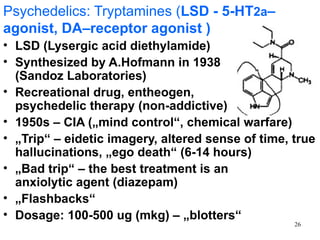
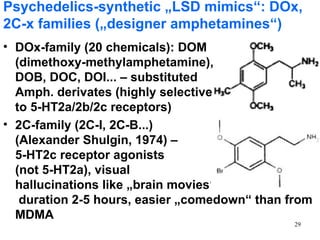
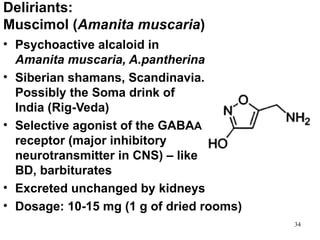
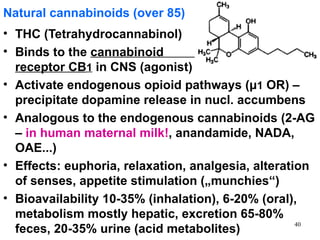
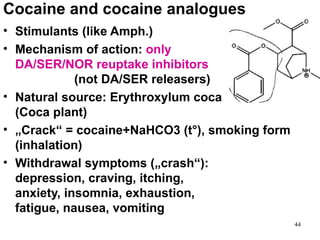
The document provides an overview of recreational drugs, focusing on their purpose, effects, and various categories including stimulants, hallucinogens, and novel substances. It highlights the complexities of drug use, including addiction risks, legal concerns, and potential neurotoxicity. Additionally, it discusses specific drugs and their mechanisms of action, as well as the syndromes associated with their toxicity.