1. The student measured the initial rates of simple and complex chemical reactions by tracking concentration changes over time. For a simple reaction of A + B → C, varying concentrations of reactants A and B did not change the positive slope of the initial rate graph.
2. A two-step reaction of A + B → C, C + D → E was also examined. Concentration graphs showed no statistical difference when varying the concentration of reactant D.
3. Finally, a reversible reaction of A + B ↔ C + D was found to reach equilibrium as predicted by chemical kinetics theory, supporting the concepts studied.
![Evans Asumadu Stimulating Life
MEASURING THE INITIAL RATE OF REACTIONS
My project focuses on measuring the initial rate of reaction in chemical reactions.
Although extensive work has been done with chemical kinetics, my project offers a whole
new perspective on how chemicals interact, the rate it interacts and conditions.
Chemical kinetics is defined as the study of the speed with which a chemical reaction
occurs and the factors that affect this speed. Knowing the speed at which a reaction occurs
helps determine the different concentrations needed for each reaction.
There are three parts to my project. The first part measures the initial rate of a simple
chemical reaction such as A + B C. For example A = [Silver (Ag+
)] and B =
[Chlorine (Cl-
)] and C = [AgCl]. When Ag+
collides with Cl-
they form AgCl. Series of data
were collected to see the correlation between initial rates of these reactions and different
concentrations.
The second part of my project measures a more complex reaction to see the effects of
time and concentration. In this reaction, A + B C, C + D E. For example A=
[CH3OH] , B= [H+], C= [CH3
+
], D= [Cl-
] and E= [CH3Cl ] When A hits B ,they turn into
C , then C will find D , when C hits D they turn into E.
In my final project, I did a reversible reaction. In this reaction A + B C + D
and C + D A + D. If everything we know about kinetics is right then they should go
into equilibrium.
1. Chemical kinetics of a reaction [ A+ B C ]
A = [Silver (Ag+)] and B = [Chlorine (Cl-)] to form C= [Silver Chloride AgCl]
I measured the initial time it took for Ag+ to collide with Cl- to form AgCl.](https://image.slidesharecdn.com/aad285ba-1759-48fd-aca4-fb7c1fce87a7-160116155355/75/Rateofreaction-1-2048.jpg)
![Evans Asumadu Stimulating Life
Figure 1b. Shows a graph of the initial rate of reaction of log R vs. log S
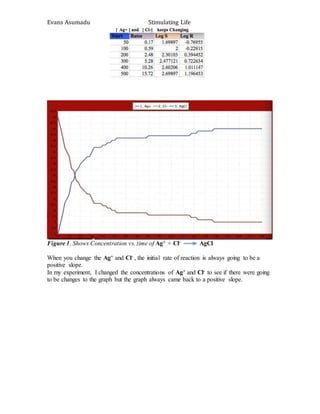
2. Chemical Kinetics of a reaction [ A + B C C+ D E ]
In this reaction A= [CH3OH] , B= [H+] , C= [CH3
+], D = [Cl-] and E= [CH3Cl]
In this reaction I did a setup of A, B and D to see the rate at which each reacts.
Figure 2. Shows Concentration vs. time of A + B = C then C + D = E
From the trials I did, there is no statically difference between changing the
concentrations of Cl- or CH3OH. This confirms the kinetics theory of having
3. Chemical kinetics of a Reverse reaction [A + B C + D and C + D A + D]
y = 1.9796x - 4.1581
R² = 0.9991
-1
-0.5
0
0.5
1
1.5
0 0.5 1 1.5 2 2.5 3
ConcentrationlogR
Log S
Initial Rate of Reaction
Log R](https://image.slidesharecdn.com/aad285ba-1759-48fd-aca4-fb7c1fce87a7-160116155355/85/Rateofreaction-3-320.jpg)
![Evans Asumadu Stimulating Life
If what we know about kinetics is correct then this reaction should be going to
equilibrium. Figure 3 definitely supports the kinetics theory.
Figure 3. Measuring the concentration vs. time in [A+B C+D] and [C+D
A+B]](https://image.slidesharecdn.com/aad285ba-1759-48fd-aca4-fb7c1fce87a7-160116155355/85/Rateofreaction-4-320.jpg)