Embed presentation
Downloaded 19 times
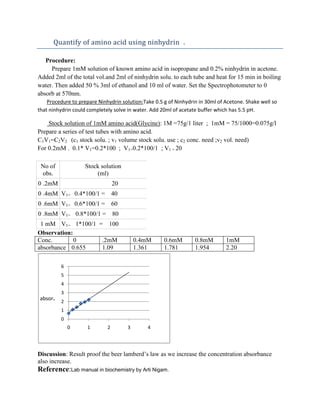


This document describes a procedure for quantifying amino acids using ninhydrin. A series of glycine standards of increasing concentration from 0.2mM to 1mM are prepared using serial dilution calculations. The standards and unknown samples are mixed with ninhydrin solution and heated to develop a color. Absorbance is measured at 570nm and a linear relationship between concentration and absorbance is observed, verifying Beer's law.

