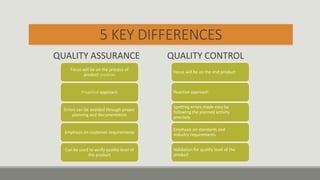
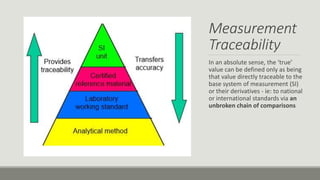
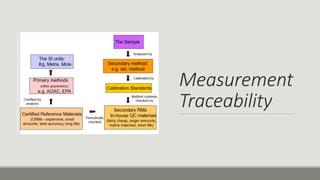
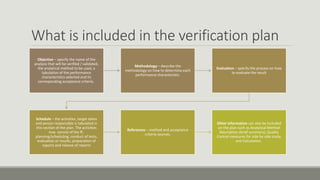
This document discusses quality assurance and quality control programs for waste water analysis laboratories. It outlines key differences between quality assurance and quality control such as their focus on process vs. product and being proactive vs. reactive. It also provides details on quality assurance documentation and measures including calibration, reference materials, and proficiency testing. For quality control, it describes validation and verification of analytical methods, determination of method detection limits, initial and ongoing demonstration of analyst capability, control charts, and corrective actions. Specific procedures are defined for determining method detection limits, accuracy, precision, linearity, and reporting verification results.