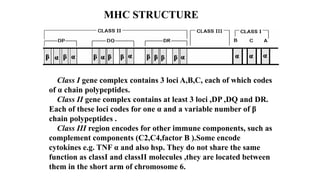
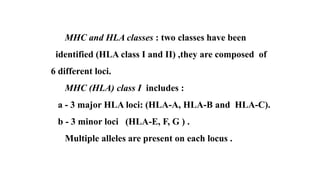
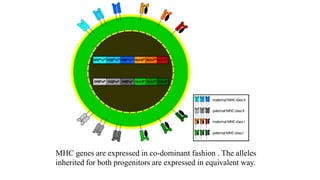
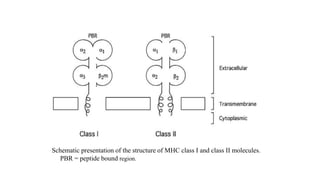
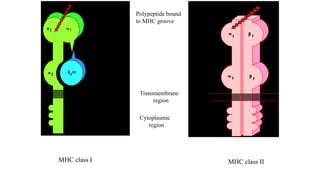
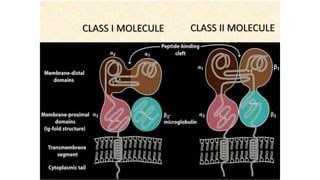
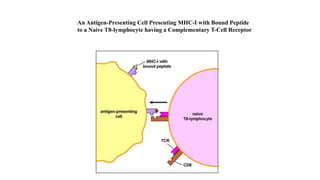
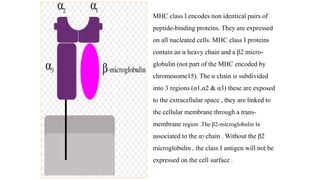
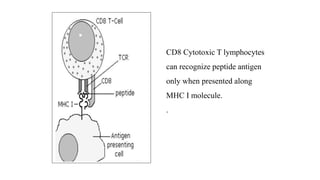
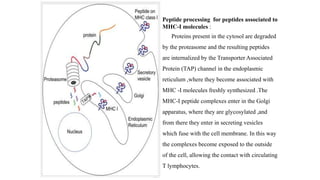
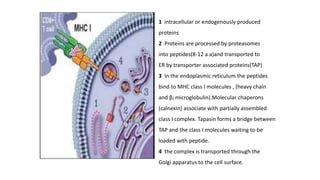
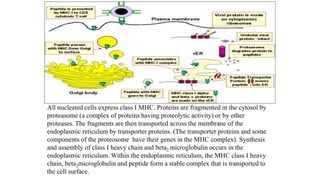
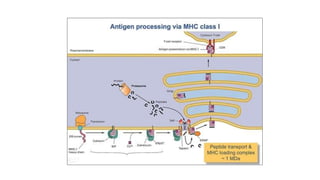
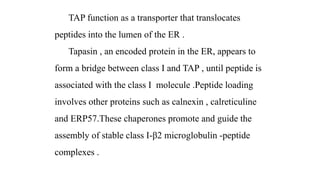
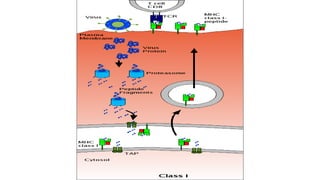
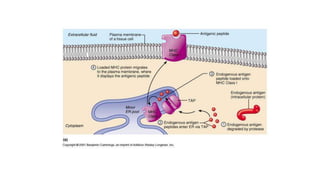
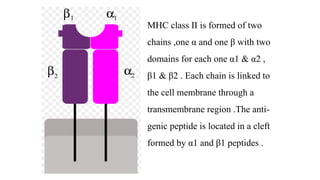
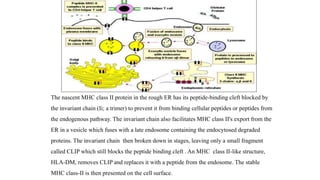
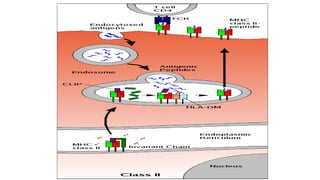
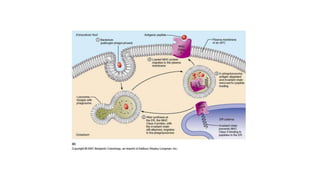
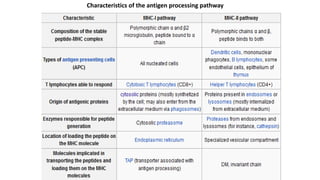
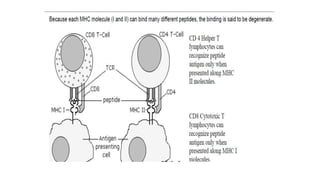
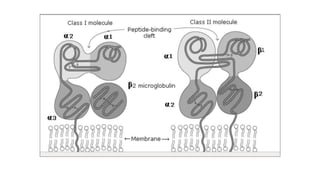
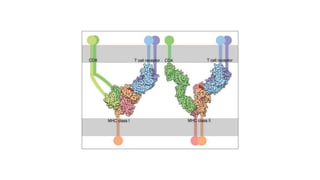
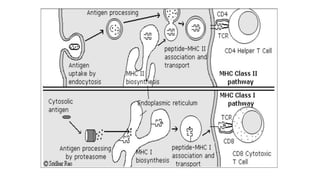
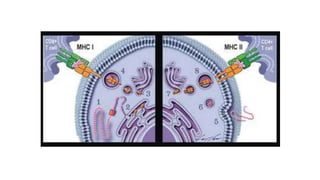
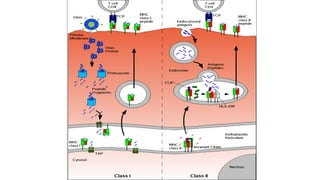
The major histocompatibility complex (MHC) is a crucial genomic region on chromosome 6 responsible for encoding molecules that help the immune system distinguish self from non-self proteins. MHC molecules are highly polymorphic, and their diversity is key for immune responses, enabling T cells to recognize and respond to infections, grafts, and tumors. They consist of class I, II, and III molecules, which have distinct structural and functional roles in antigen presentation and immune regulation.