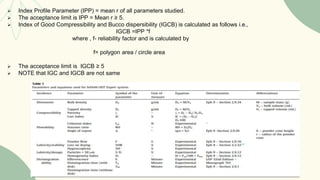
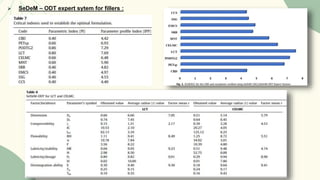
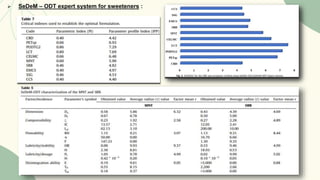
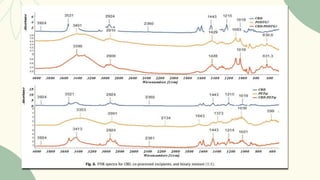
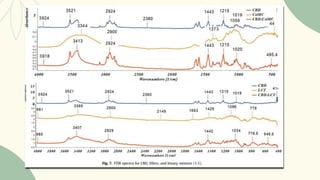
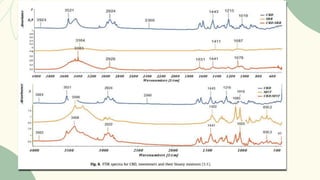
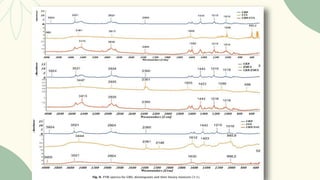
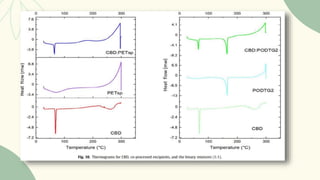
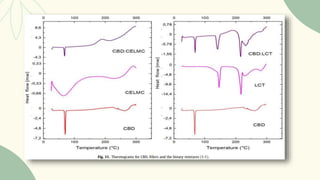
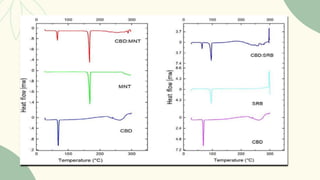
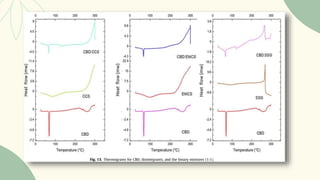
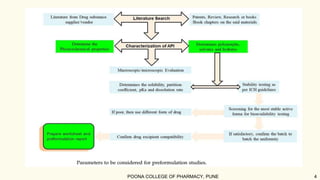
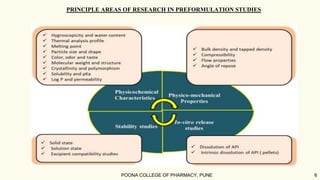
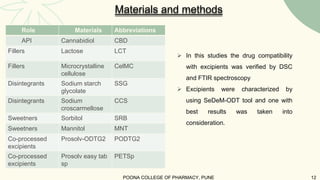
The document outlines preformulation studies of tablets focused on the physicochemical properties of drug candidates, particularly cannabidiol (CBD), to enhance the development of stable and bioavailable dosage forms. It discusses key areas such as organoleptic properties, bulk characterization, solubility, and stability analysis to guide the selection of excipients for developing orodispersible tablets. A case study demonstrates methodology and results, emphasizing the compatibility of CBD with various excipients and the effectiveness of using specific formulations for improved disintegration and performance.









![Drug cannabidiol
IUPAC name 2-[(1R,6R)-3-methyl-6-prop-
1-en-2-ylcyclohex-2-en-1-
yl]-5-pentylbenzene-1,3-
diol
Molecular formula C21H30O2
Molecular weight 314.5g/mol
BCS classification Type II
Solubility 0.7 µg/mL
Melting point 66 °C (151 °F)
Drug class hallucinogen
DRUG PROFILE
POONA COLLEGE OF PHARMACY, PUNE 13](https://image.slidesharecdn.com/finalppt-221126174216-4e69804d/85/preformulation-studies-by-SeDeM-expert-system-tool-13-320.jpg)