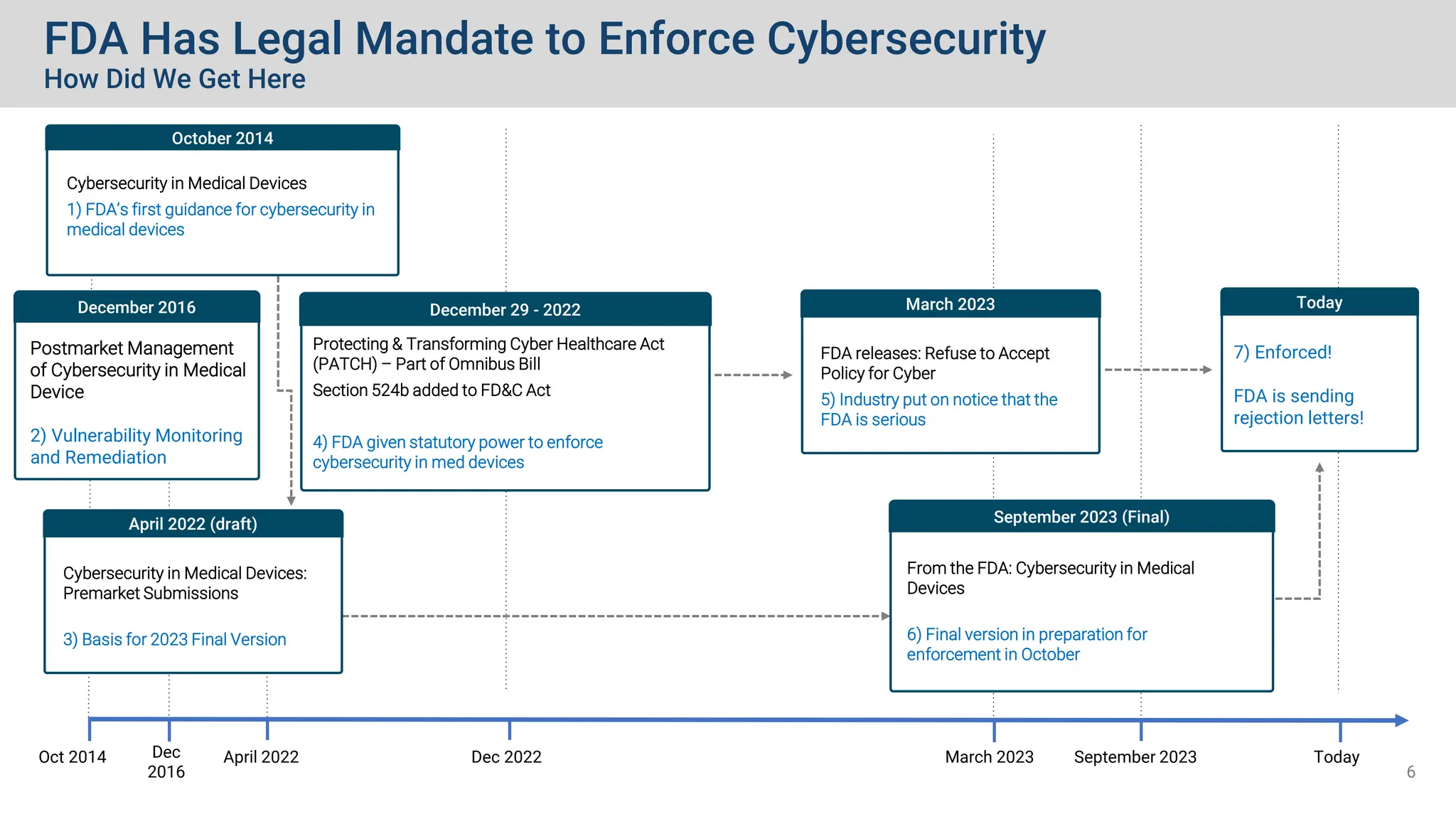
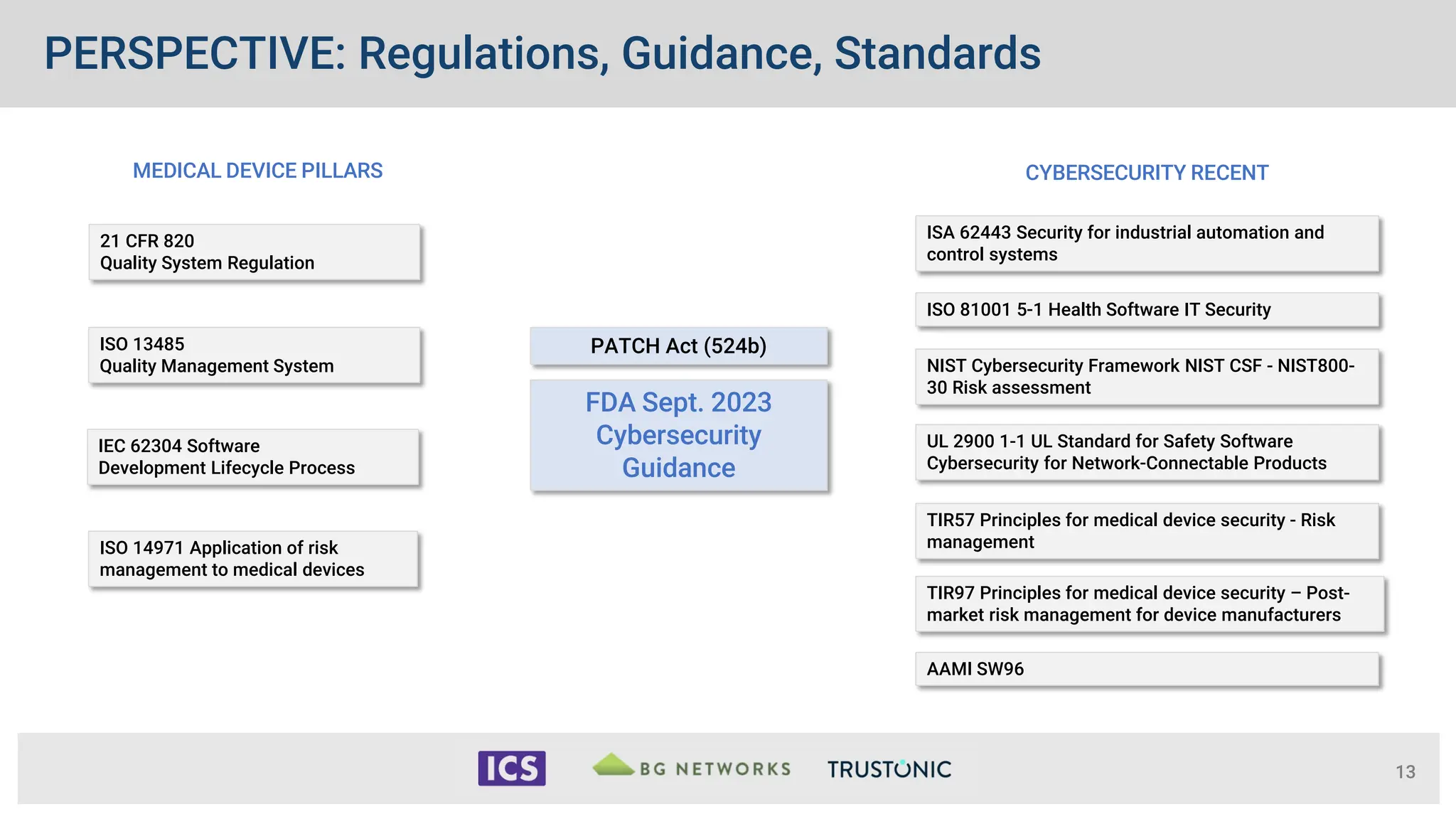
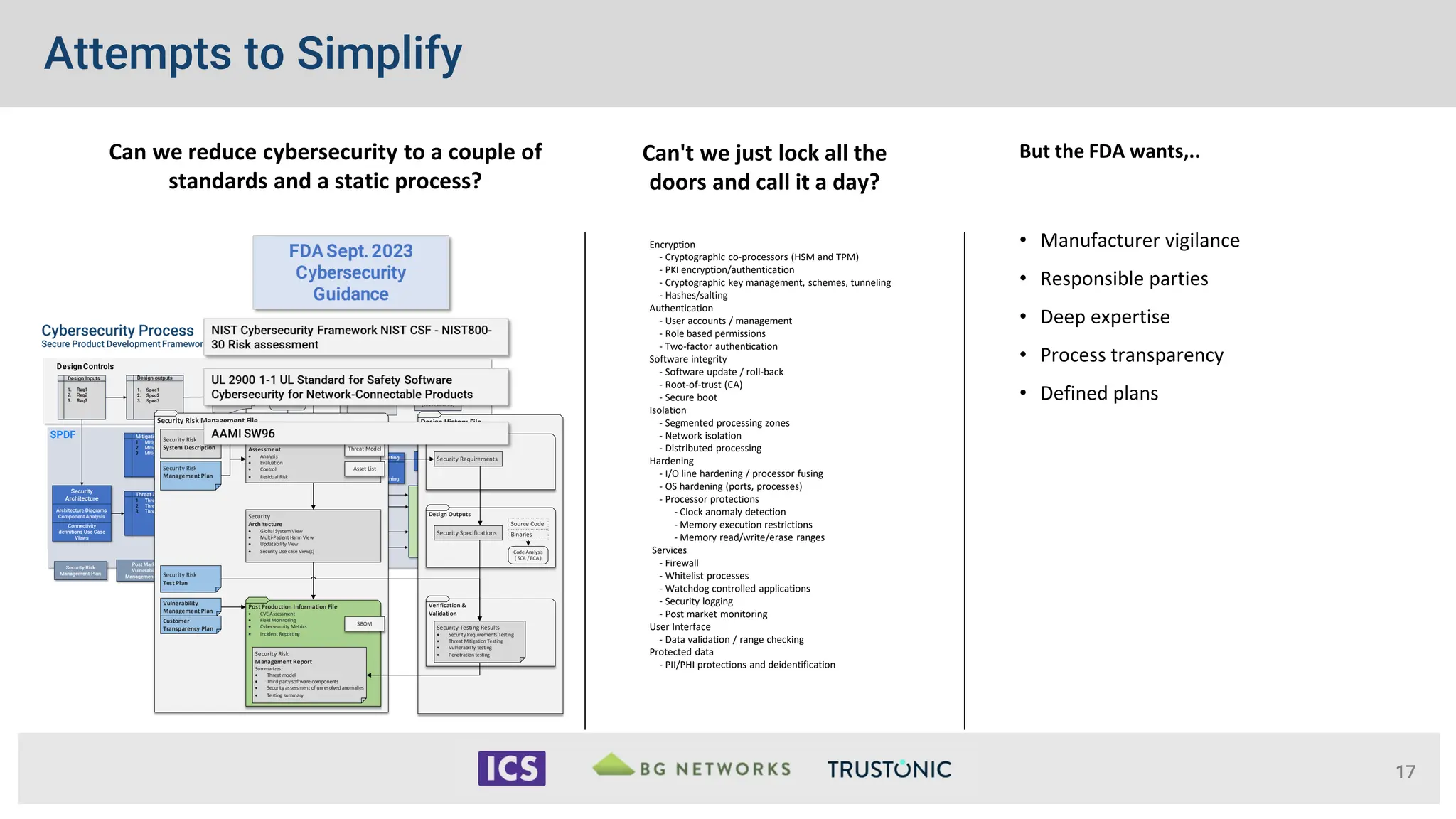
The document outlines the essential cybersecurity requirements for medical devices as mandated by the FDA, emphasizing a secure product development framework and the need for thorough risk assessment and threat modeling. It discusses compliance with various standards, the importance of software bills of materials (SBOMs), and specific cybersecurity testing protocols that manufacturers must follow. The document also highlights the evolving nature of regulatory authority and enforcement concerning cybersecurity in medical devices since the FDA's first guidance in 2014.