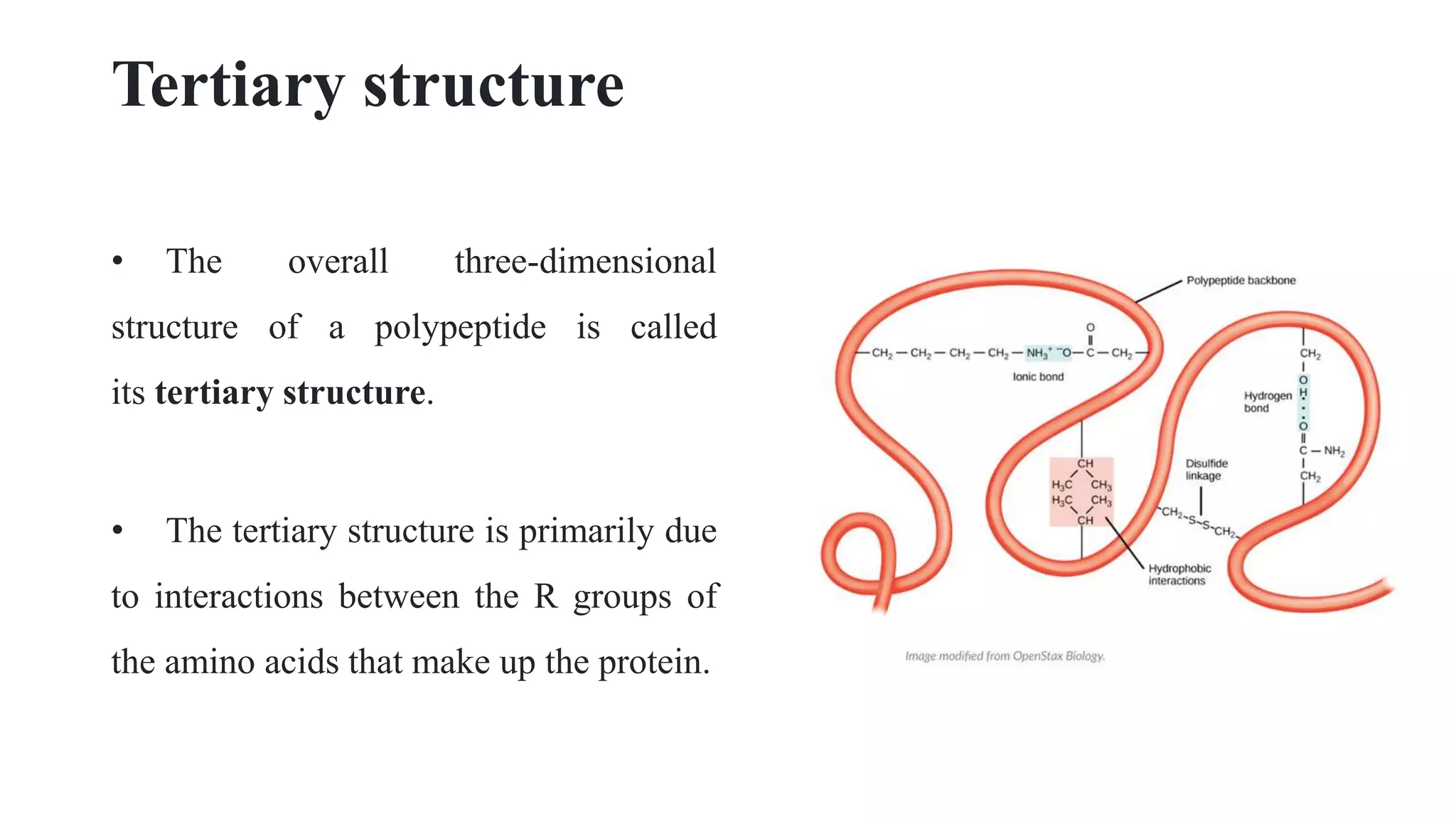
This document discusses the structure of proteins at multiple levels. It begins by defining proteins and their functions in the body. It then describes the primary, tertiary, and quaternary structure of proteins. The primary structure is the linear sequence of amino acids. Tertiary structure results from interactions between amino acid side chains that give the overall 3D shape. Quaternary structure occurs when multiple protein subunits combine, as seen in hemoglobin. Non-covalent bonds like hydrogen bonding contribute to tertiary and quaternary structure formation.








![Reference
1. Khan, R. H., Siddiqi, M. K., & Salahuddin, P. (2017). Protein structure and function. Basic
biochemistry, 5, 1-39.
2. Berg, J. M., Tymoczko, J. L., and Stryer, L. (2002). Tertiary structure: Water-soluble proteins fold
Into compact structures with nonpolar cores. In Biochemistry (5th ed., section 3.4). New York,
NY: W. H. Freeman. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK22375/.
3. 3. Berg JM, Tymoczko JL, Stryer L (2002). "Section 3.5Quaternary Structure: Polypeptide Chains
Can Assemble Into Multisubunit Structures". Biochemistry (5. ed., 4. print. ed.). New York, NY
[u.a.]: W. H. Freeman. ISBN 0-7167-3051-0.](https://image.slidesharecdn.com/pptproteinstructure-220912042226-797d638b/75/PPT-Protein-Structure-pptx-10-2048.jpg)