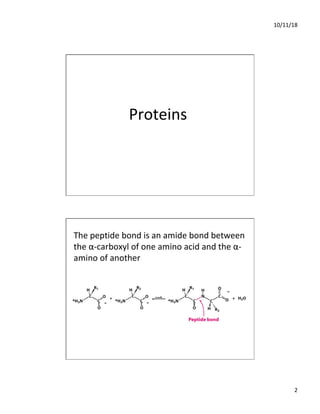
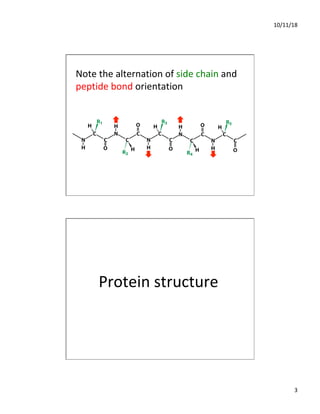
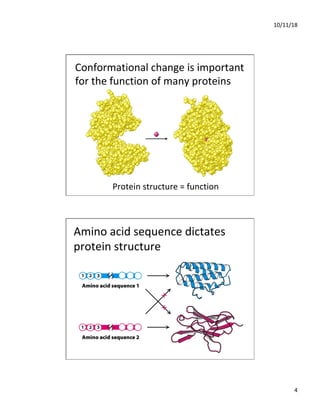
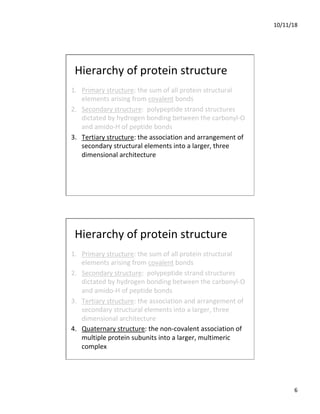
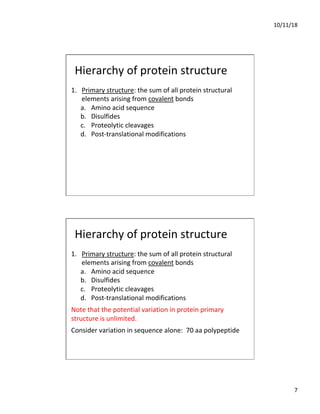
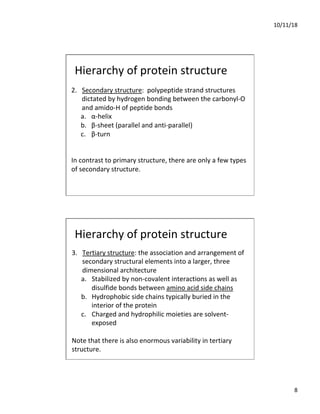
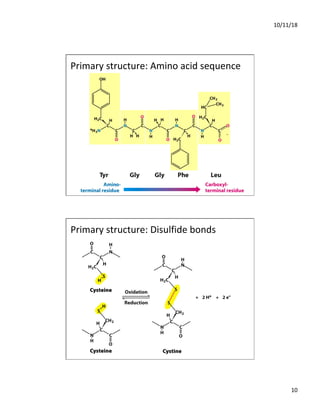
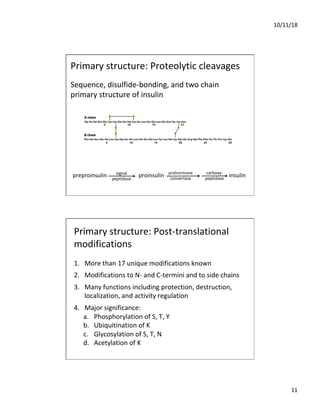
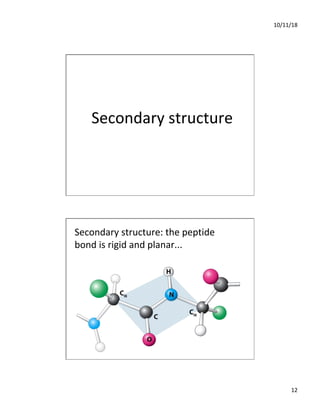
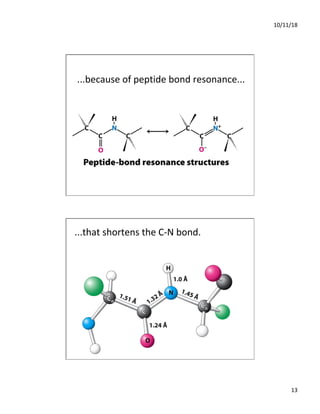
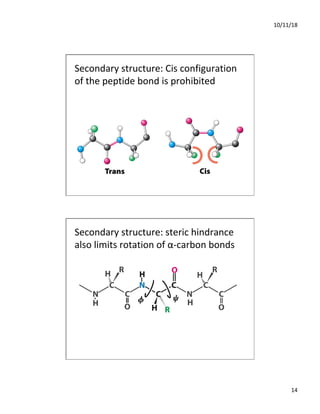
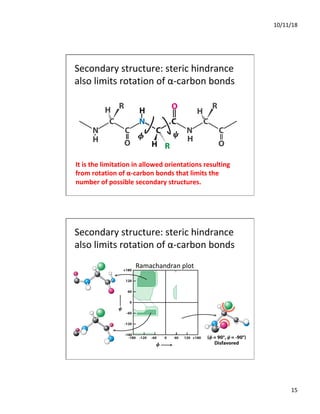
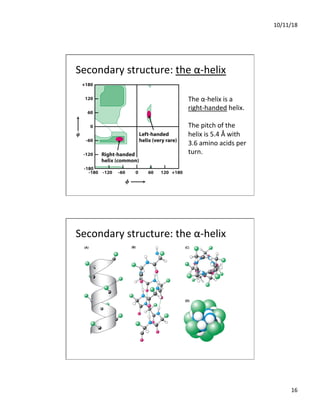
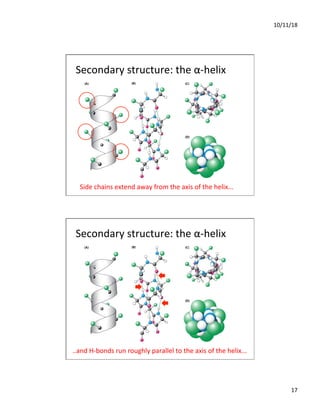
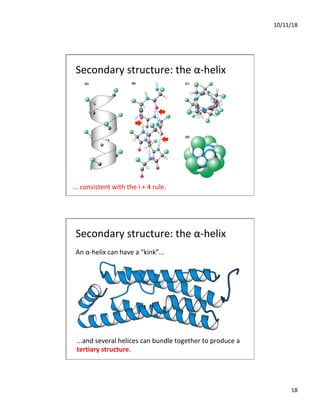
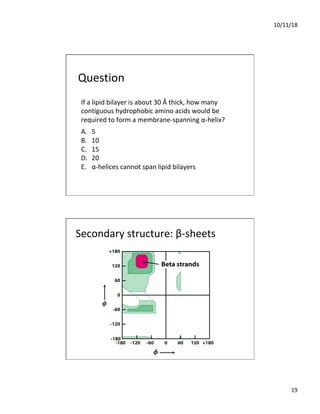
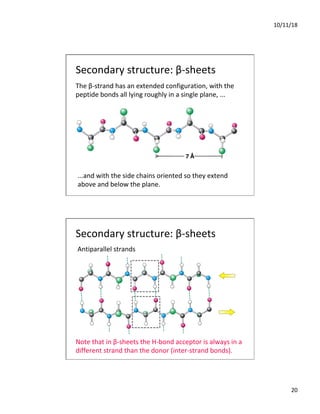
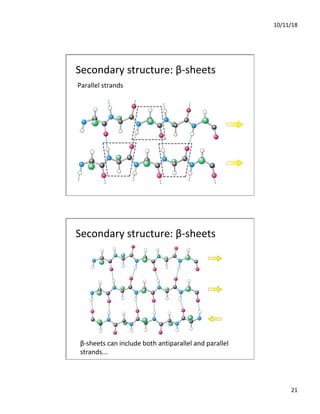
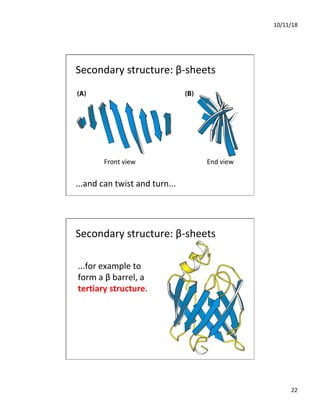
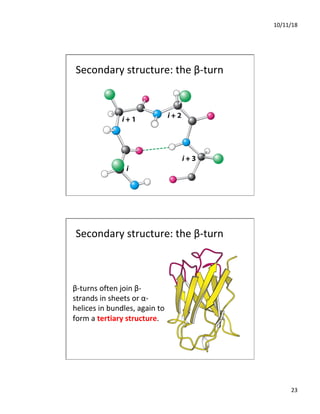
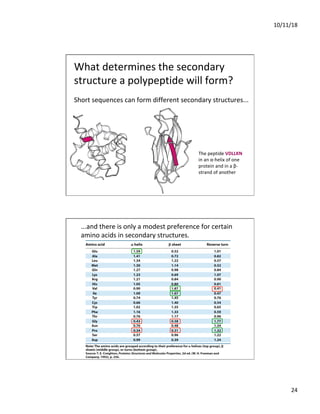
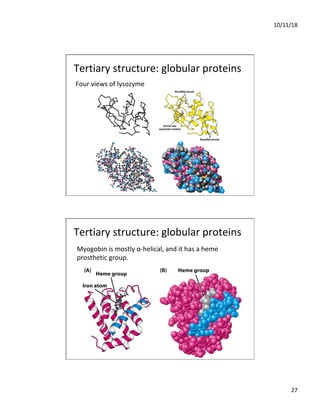
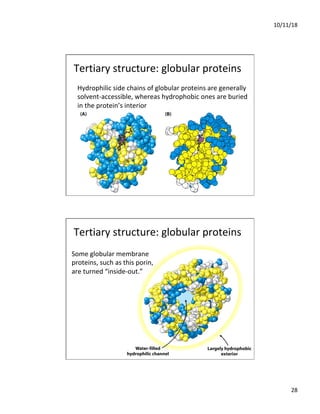
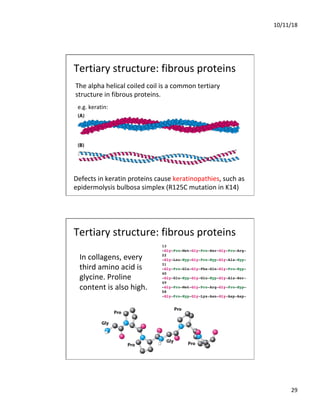
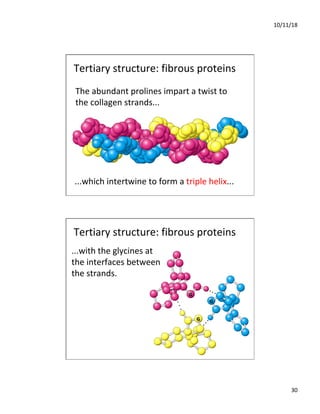
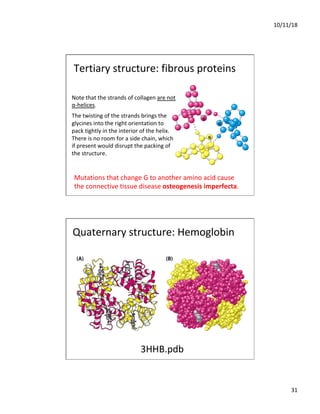
The document discusses the hierarchy of protein structure including primary, secondary, tertiary, and quaternary levels, detailing how amino acid sequences and chemical bonds contribute to protein conformation and function. Primary structure arises from covalent bonds, secondary structure is formed by hydrogen bonding, and tertiary structure involves the 3D arrangement of these elements. Quaternary structure represents the association of multiple protein subunits, crucial for functional regulation.