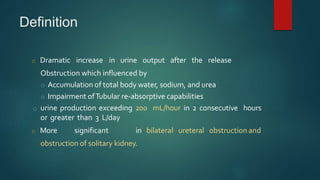
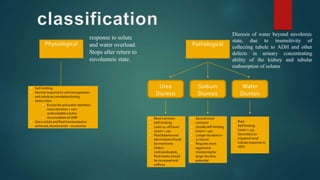
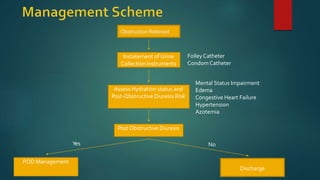
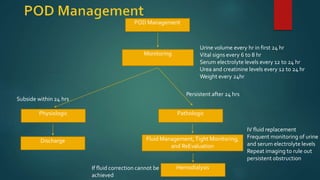
Post-obstructive diuresis occurs after the release of urinary tract obstruction and is caused by the release of sodium, water, and urea that accumulated during the period of obstruction. It can be physiological and self-limiting, lasting less than 24 hours with urine output over 200 mL/hour, or pathological, persisting beyond 24 hours. Pathological post-obstructive diuresis is due to decreased tubular reabsorption and impaired urinary concentration caused by loss of the medullary gradient from obstruction. Careful monitoring of fluid balance and electrolytes is needed to prevent complications of volume depletion.