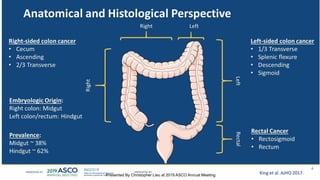
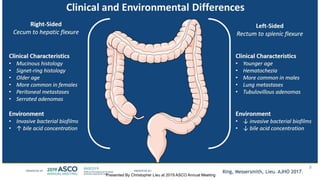
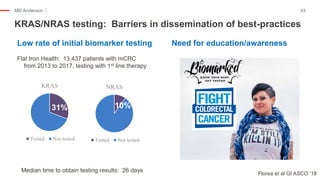
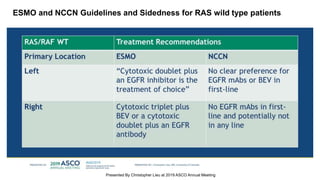
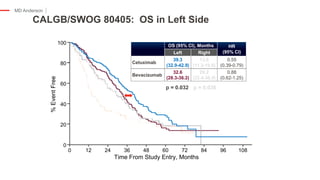
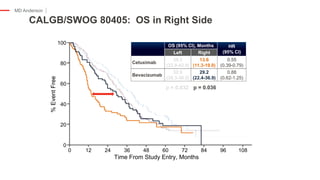
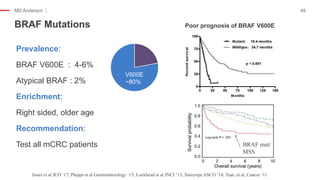
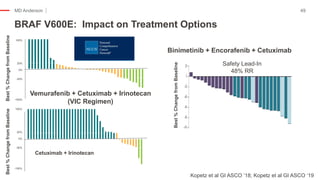
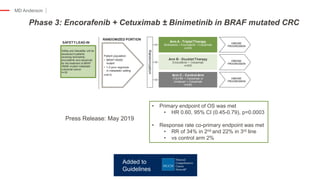
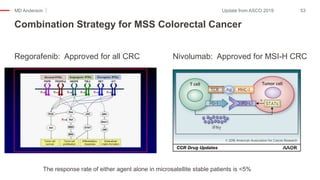
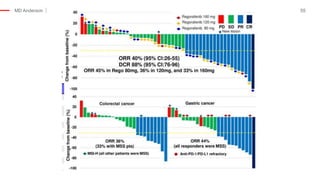
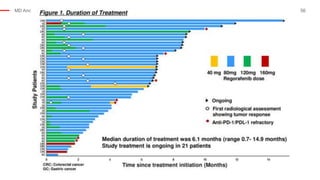
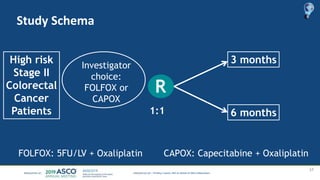
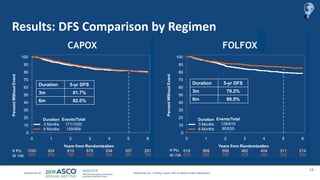
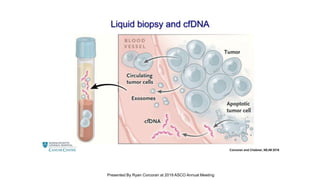
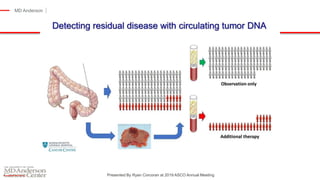
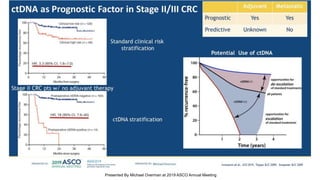
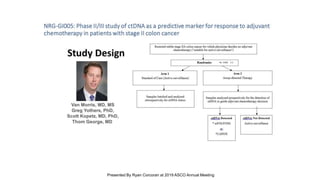
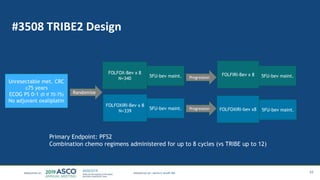
The document outlines updates from the ASCO 2019 conference on colorectal cancer treatment strategies, emphasizing discussions on neoadjuvant therapy, the duration of chemotherapy, and the implications of tumor location on treatment outcomes. Key findings indicate that three months of capox is sufficient for most high-risk stage II and low-risk stage III patients, along with advancements in liquid biopsy technology for detecting residual disease. The presentation is led by Dr. Scott Kopetz, a noted expert in the field, highlighting the need for tailored treatment regimens based on individual patient characteristics.









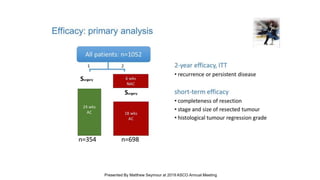

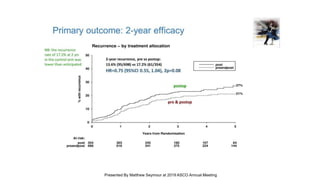












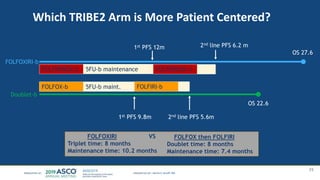
![Median follow up =
30.6 mos
Arm A
N = 340
Arm B
N = 339
Events, N (%) 217 (64%) 191 (56%)
Median OS, mos 22.6 27.6
HR = 0.81 [95%CI: 0.67-0.98] p=0.033
Overall Survival – preliminary results](https://image.slidesharecdn.com/junewebinarcombined-190625191324/85/Post-ASCO-Webinar-2019-33-320.jpg)