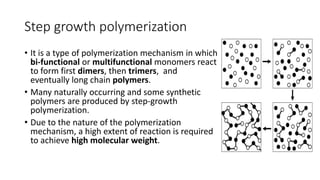
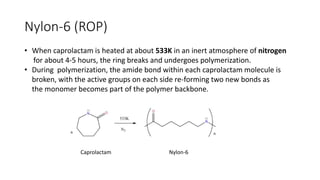
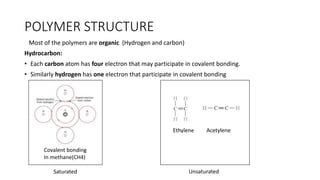
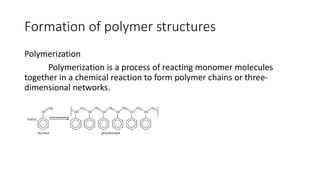
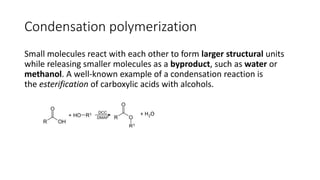
Polymeric materials are formed through polymerization reactions that link together small molecular units into long chains or networks. There are three main types of polymerization: condensation polymerization, step-growth polymerization, and ring-opening polymerization. Condensation polymerization involves monomers reacting to form larger units while releasing smaller molecules as byproducts. Step-growth polymerization proceeds through the formation of dimers, trimers, and eventually long chains. Ring-opening polymerization breaks cyclic monomers open to add them to the growing polymer chain. Examples provided include the condensation polymerization of polyethylene terephthalate and the ring-opening polymerization of nylon-6 from caprolactam.

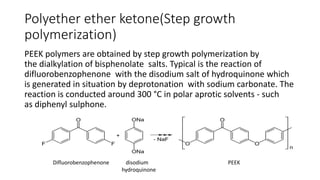
![Polyethylene terephthalate(condensation
polymerization)
• Polyethylene terephthalate is produced
from ethyleneglycol and dimethalterephthalate(DMT)
(C6H4(CO2CH3)2) or terephthalic acid.
• The former is a transesterification reaction, whereas the
latter is an esterification reaction.
The reactions are idealized as follows:
First step
C6H4(CO2CH3)2 + 2 HOCH2CH2OH → C6H4(CO2CH2CH2OH)2 + 2 CH3OH
DMT + ethylene glycol terephthalic acid + methanol
Second step
n C6H4(CO2CH2CH2OH)2 → [(CO)C6H4(CO2CH2CH2O)]n + n HOCH2CH2OH
terephthalic acid PET + ethylene glycol](https://image.slidesharecdn.com/maharajan2017214030polymers-180706022641/85/Polymeric-materials-Formation-of-polymer-structure-6-320.jpg)